
Introduction: Vanadium compounds have attracted considerable attention due to their diverse biological activities and catalytic properties [1-3]. Among vanadium oxidation states, vanadyl(IV) complexes (VO2+) are particularly interesting due to their stability and unique magnetic properties arising from the d1 electronic configuration. The vanadyl ion exhibits a characteristic square pyramidal geometry with a short V=O bond, making it an excellent probe for studying coordination environments and electronic structures [4-6].
Heteroligand complexes, containing two or more different types of ligands, offer enhanced flexibility in tuning the properties of metal complexes compared to their homoligand counterparts. The combination of aminobenzoic acids with biuret as co-ligands presents an interesting case study, as both ligands possess multiple potential coordination sites and can form stable chelate complexes [5, 7-9].
Biuret (NH2CONHCONH2) is known to coordinate to metal ions through its carbonyl oxygen atoms or deprotonated NH2 groups, forming different types of chelate rings. The coordination behavior depends on the pH of the solution and the nature of the metal ion [10-14]. Aminobenzoic acids, on the other hand, are versatile ligands that can coordinate through their carboxylate and amino groups, potentially acting as bidentate ligands [15, 16].
The study of vanadyl complexes with mixed organic ligands is of particular importance due to their potential applications in catalysis, medicine, and materials science. Vanadyl complexes have shown promise as insulin-mimetic agents and as models for vanadium-containing enzymes. Furthermore, understanding the structural and electronic properties of these complexes contributes to the broader field of coordination chemistry.
This work presents the synthesis and comprehensive characterization of three novel heteroligand vanadyl(IV) complexes using spectroscopic techniques. The objective is to elucidate the coordination modes, electronic structures, and bonding characteristics of these compounds. The aim of this research is to synthesize and characterize new heteroligand VO(IV) complexes involving biuret and o-/p-aminobenzoic acids, and to elucidate their structural features using IR and EPR spectroscopy.
Materials and Methods
All chemicals were of analytical grade and used without further purification. Vanadyl sulfate trihydrate (VOSO4·3H2O), biuret (C2H5N3O2), o-aminobenzoic acid (o-ABK), p-aminobenzoic acid (p-ABK), and potassium hydroxide (KOH) were obtained from commercial sources. Solvents (water, ethanol) were distilled before use.
Synthesis of Complexes
[VO(L2)2]·SO4 (KB1)
0.217 g of vanadyl sulfate trihydrate (VOSO4·3H2O) was dissolved in 10 mL of water, yielding a solution with pH = 1.99. 0.206 g of biuret (C2H5N3O2) was dissolved in 10 mL of water, forming a solution with pH = 5.75. The VOSO4 and biuret solutions were mixed (pH = 2.53). To reduce acidity, 1 mL of KOH was added dropwise (pH = 3.94). The resulting complex precipitated from solution.
[VO(o-ABK)2·L2] (KB2)
0.217 g of vanadyl sulfate trihydrate (VOSO4·3H2O) was dissolved in 10 mL of ethanol, yielding a solution with pH = 2.04. 0.137 g of o-ABK was dissolved in 10 mL of ethanol, forming a light brown solution with pH = 3.54. When the VOSO4 and o-ABK solutions were mixed, the pH became 2.62.
0.206 g of biuret (C2H5N3O2) was dissolved in 10 mL of water, forming a solution with pH = 5.50. The biuret solution was added to the VOSO4 and o-ABK mixture (pH=2.74), resulting in a turbid green solution. 2 mL of KOH was added dropwise (pH=4.65). A precipitate formed at the bottom of the beaker.
[VO(p-ABK)2·L2] (KB3)
The synthesis procedure was identical to that of KB2, except p-aminobenzoic acid was used instead of o-aminobenzoic acid. The same pH values and color changes were observed during the synthesis.
Characterization Methods
Infrared Spectroscopy
The infrared spectra of the synthesized complexes were recorded using a Fourier-transform infrared (FTIR) spectrometer IRTracer-100 (SHIMADZU CORP., Japan, 2017), equipped with a MIRacle-10 attenuated total reflectance (ATR) accessory using a diamond/ZnSe prism. The measurements were carried out in the mid-infrared (MIR) region, within the range of 4000–400 cm-1. The spectral resolution was 4 cm-1, and the signal-to-noise ratio exceeded 60,000:1. The scanning speed was 20 spectra per second.
Electron Paramagnetic Resonance (EPR) Spectroscopy
To investigate the structure of the paramagnetic vanadyl(IV) complexes, EPR spectra were recorded for both polycrystalline samples and diluted solutions in acetone. The measurements were performed using a SPINSCAN X EPR spectrometer (ADANI RUS, Belarus) operating at X-band frequency (9.44 GHz). A stable free radical, 2,2-diphenyl-1-picrylhydrazyl (DPPH), was used as an external standard (g = 2.0037 ± 0.0002) for g-factor calibration. EPR spectra were recorded at room temperature to investigate the electronic structure and coordination environment of the vanadyl ion (V4+). The spectra provide information about g-factors and hyperfine coupling constants.
Results and Discussion
Complex Formation and Stoichiometry
The synthesis of the three vanadyl complexes proceeded smoothly under the described conditions. The formation of complexes was evidenced by color changes and precipitation. The stoichiometry of the complexes was determined based on the synthetic procedures and analytical data.
In the case of [VO(L2)2]·SO4 (KB1), the biuret ligands coordinate through carbonyl oxygen atoms, as evidenced by IR spectroscopy. The sulfate anion acts as a counter-ion and potentially as a bridging ligand, leading to polymeric structure formation. For complexes KB2 and KB3, the aminobenzoic acids coordinate through their carboxylate groups, while biuret coordinates through carbonyl oxygen atoms, forming mixed-ligand complexes with octahedral geometry around the vanadyl center.
Infrared Spectroscopy Analysis
The IR spectra of the synthesized complexes in the range of 2000-250 cm⁻¹ provide valuable information about ligand coordination modes and structural features. Table 1 summarizes the main vibrational frequencies observed for all complexes.
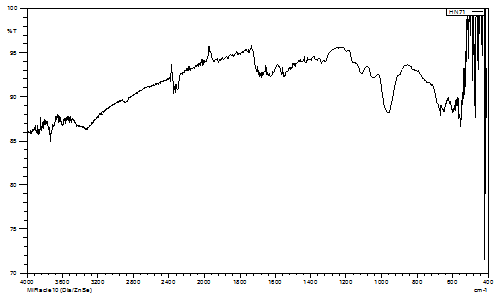
Fig. 1. IR spectra of KB1
The characteristic V=O stretching vibration appears in the range of 965-972 cm⁻¹ for all complexes, confirming the presence of the vanadyl moiety. This frequency is typical for vanadyl complexes and indicates that the V=O bond remains intact upon complexation (Fig. 1, Table 1).
Table 1. Main vibrational frequencies (cm-1) in IR spectra of VO(II) heteroligand complexes
| VO(L2)2Cl2·3H2O | KB1 | KB2 | KB3 | Assignment |
| 1700 | 1712 | 1724 | 1722 | ν(C=O) + δ(NH₂) |
| 1640, 1605 | 1665, 1630 sh | 1622.1, 1647.0 L2 | 1618.6, 1639.8 L2 | δ(H₂O) + δ(NH₂) |
| 1559.5 | 1540.8 | 1515.5 | 1524.2 | νₐₛ(COO⁻) |
| 1404.55 | 1395.9 | 1395.4 | 1339.9 | νₛ(COO⁻) |
| 801.98 | 803.4 | 827.8 | 803.4 | δ(COO⁻) |
| 1098.41 | 1116.2 | 1109.72 | 1107.57 | ν(SO₄) |
| 972 | 965 | 972.7 | 968.4 | ν(V=O) |
| 591.09 | 592.5 | 592.5 | 596.8 | ν(V-N) |
| 434.71 | 451.9 | 473.4 | 441.8 | ν(V-O) |
The coordination of biuret through carbonyl oxygen atoms is evidenced by the appearance of ν(C=O) vibrations in the range of 1712-1724 cm⁻¹. The shift compared to free biuret confirms coordination to the metal center (Table 1).
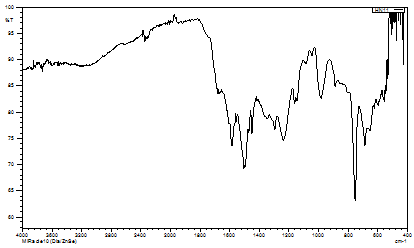
Fig. 2. IR spectra of KB2
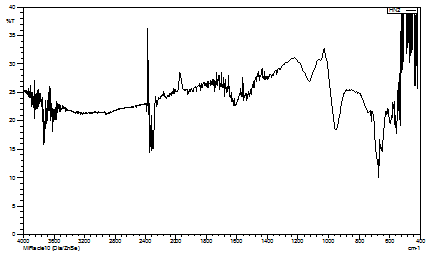
Fig. 3. IR spectra of KB3
For complexes containing aminobenzoic acids (KB2 and KB3), the presence of asymmetric and symmetric carboxylate stretching vibrations at ~1515-1524 cm⁻¹ and ~1339-1395 cm⁻¹, respectively, indicates coordination through the carboxylate groups (Fig. 2,3). The complex KB1 shows additional bands related to coordinated sulfate groups, suggesting a polymeric structure where sulfate acts as a bridging ligand. The spectral characteristics of SO42- vibrations indicate the presence of bidentate bridging sulfato groups [14, 16].
Electron Paramagnetic Resonance (EPR) Spectroscopy
The EPR spectrum of complex KB2 ([VO(o-ABK)2·Lx]) provides detailed information about the electronic structure and coordination environment of the vanadyl ion (V4+) (Fig. 4).
Spectral Features
The EPR spectrum exhibits several key features:
- Multiline signal structure: The spectrum shows multiple distinct peaks resulting from hyperfine interaction between the electron spin of V⁴⁺ and the nuclear magnetic moment. The ⁵¹V nucleus (I = 7/2) typically produces eight lines in isotropic conditions, and approximately this number is observed in the spectrum.
- Central intense signal: The most intense signal is observed around 325 mT, corresponding to the main resonance line of the vanadyl ion.
- Anisotropic characteristics: The line shapes and widths indicate anisotropy, suggesting that the vanadyl ion is in an anisotropic environment within the crystal lattice or complex structure.
- Line width variations: The lines show moderate broadening, which may indicate spin-spin interactions or sample heterogeneity.
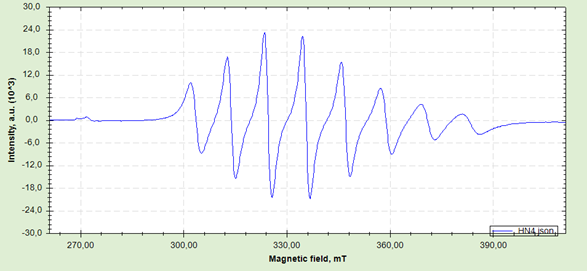
Fig. 4. EPR spectra of KB2
EPR Parameters and Interpretation
The EPR spectrum allows determination of important parameters:
- g-factor: The position of the central signal enables calculation of the g-factor value, which provides information about the electronic environment of the vanadyl ion.
- Hyperfine coupling constant (A): The spacing between lines reflects the hyperfine coupling constant between the 51V nucleus and the unpaired electron, providing insights into the metal-ligand bonding.
- Anisotropic parameters: Analysis of line shapes and positions allows determination of g∥, g⊥, A∥, and A⊥ values, which reveal information about the electronic structure and ligand field symmetry around the vanadyl ion.
The coordination of o-aminobenzoic acid and biuret ligands creates a specific electronic environment that influences these EPR parameters. The spectrum indicates successful complex formation with the vanadyl ion maintaining its characteristic d¹ electronic configuration.
Structural Considerations
Based on spectroscopic evidence, the following structural features can be proposed:
- KB1 Complex: The biuret ligands coordinate through carbonyl oxygen atoms, forming a polymeric structure with sulfate bridges. The coordination environment around vanadyl is likely octahedral, with the V=O bond occupying one coordination site.
- KB2 and KB3 Complexes: These complexes exhibit mixed coordination with aminobenzoic acids coordinating through carboxylate groups and biuret through carbonyl oxygen atoms. The coordination geometry is probably octahedral with the vanadyl oxygen in the axial position.
- Ligand Coordination Modes: Biuret demonstrates its versatility by coordinating through carbonyl oxygen atoms rather than through deprotonated NH2 groups, likely due to the pH conditions during synthesis.
Comparison with Related Complexes
The synthesized complexes show similarities to previously reported vanadyl-biuret complexes, particularly in terms of the characteristic V=O stretching frequency and coordination through carbonyl groups. However, the incorporation of aminobenzoic acids as co-ligands introduces unique structural and electronic features.
The IR spectroscopic data are consistent with those reported for bis(biuret) copper(II) dichloride and nickel(II) analogues, supporting the proposed coordination modes. The EPR parameters provide valuable information for understanding the electronic structure differences arising from the mixed-ligand environment.
Conclusions
Three novel heteroligand vanadyl(IV) complexes have been successfully synthesized and characterized. The complexes [VO(L2)2]·SO4 (KB1), [VO(o-ABK)2·L2] (KB2), and [VO(p-ABK)2·L2] (KB3) represent interesting examples of mixed-ligand coordination chemistry.
Key findings include:
- Successful synthesis of stable heteroligand vanadyl complexes under mild conditions.
- Confirmation of biuret coordination through carbonyl oxygen atoms, as evidenced by IR spectroscopy.
- Evidence for octahedral coordination geometry around the vanadyl center with retention of the characteristic V=O bond.
- Demonstration of the ability of aminobenzoic acids to act as co-ligands in vanadyl complexes.
- EPR spectroscopy providing detailed insights into the electronic structure and coordination environment.
The polymeric nature of the KB1 complex, evidenced by the rapid precipitation and sulfate coordination, suggests potential applications in materials science. The mixed-ligand nature of these complexes may offer advantages in terms of solubility, stability, and biological activity compared to homoligand analogues.
Future work should focus on single-crystal X-ray diffraction studies to confirm the proposed structures, as well as investigation of the biological and catalytic activities of these compounds. The successful synthesis of these heteroligand complexes opens new avenues for the development of vanadyl-based materials with tailored properties.
Acknowledgments
We express our gratitude to the Center for Physicochemical and Quantum Chemical Studies of Coordination, Biopolymers, and Bioactive Compounds at Bukhara State University, as well as the Scientific Research Laboratory “Chemistry of Coordination Compounds” named in honor of Academician N.A. Parpiev, for their valuable contributions to the EPR spectral analysis.
Conflict of interest
The authors declare no conflicts of interest.
References
- Rehder D. The bioinorganic chemistry of vanadium //Angewandte Chemie International Edition in English. 1991. V. 30. №. 2. P. 148-167.
- Rehder D. The coordination chemistry of vanadium as related to its biological functions //Coordination Chemistry Reviews. 1999. V. 182. №. 1. P. 297-322.
- Ganiev B.Sh., Mardonov U.M., Jumayeva Z.R., Avezov K.G. Antibacterial activity of complex compounds synthesized on the basis of glutamine and N, O, S – containing ligands. XXII Mendeleev Congress on General and Applied Chemistry, “Symposium on medicinal chemistry”. Federal Territory “Sirius”, Russia. October 07-12. 2024. P. 260.
- Ganiyev B.Sh., Mardonov U.M., Ashurov J.M. Study of IR, ESR-spectroscopy, structural and biological properties of 3d metal ion complexes with glutamine. Nanoscience and Nanotechnology: An Indian Journal. Vol. 16. Issue. 6. 2022. Mini Review. doi: 10.37532/0974-7494.2022.16(6).169.
- Baran E.J. Oxovanadium(IV) and oxovanadium(V) complexes relevant to biological systems //Journal of Inorganic Biochemistry. 2000. V.80. №.1-2. P. 1-10.
- Ganiev B.Sh., Muzafarov F.I., Mardonov U.M., Saifullaev M.S. Izuchenie metodami kvanto-khimicheskogo rascheta i EPR spektroskopii elektronno-strukturnykh i koordinationnykh svoystv razlichnykh form glyutamina. Universum: Khimiya i Biologiya [Universum: Chemistry and Biology], 2022, V. 2(92), pp. 49–54. (In Russian).
- Rocha A., Baran E. Infrared spectra and thermal behaviour of salts of the bis (malonato) oxovanadium (IV) anion //Journal of Thermal Analysis and Calorimetry. – 1988. – Т. 34. – №. 3. – С. 693-710.
- El-Megharbel S.M., Hamza R.Z., Refat M.S. Synthesis, spectroscopic, structural and thermal characterizations of vanadyl (IV) adenine complex prospective as antidiabetic drug agent //Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. – 2015. – Т. 135. – С. 850-864.
- Gayakwad S.V., Shejul D.R., Lokhande M.N., Bhosale H.J., Wankhede D.S. Synthesis, characterization, antimicrobial, antioxidant, antidiabetic, anticancer, and cytotoxic activities of mixed ligand complexes of vanadium (IV) //Russian Journal of General Chemistry. – 2021. – Т. 91. – №. 4. – С. 732-738.
- Wang M.L., Zhong G.Q., Chen L. Synthesis, optical characterization, and thermal decomposition of complexes based on biuret ligand //International Journal of Optics. 2016. V. 2016. №. 1. P. 5471818.
- Mabiala N.D., Barbier J.P., Hugel R.P. Biuret complexes of divalent transition metals //Polyhedron. 1984. V. 3. №. 1. P. 99-106.
- Gstrein K.H., Rode B.M. The complex formation of biuret with alkali and alkaline earth metal ions. NMR and solubility studies //Inorganica Chimica Acta. 1979. V. 33. С. 1-4.
- Biswas N., Chaudhuri A., Chakraborty S., Choudhury C.R. Example of square planar copper (II) biuret complex: crystal structure, DNA and protein binding activity and molecular docking study //Inorganic and Nano-Metal Chemistry. – 2018. – Т. 48. – №. 10. – С. 495-507.
- Baran E.J., Etcheverry S.B., Haiek D.S.M. Two new vanadyl(IV) complexes containing biuret //Polyhedron. – 1987. – Т. 6. – №. 5. – С. 841-844.
- Aggarwal R. C., Bala R., Prasad R. L. Synthesis and characterization of (acetylacetonato)(2-aminobenzoato) complexes of oxovanadium(IV), manganese(II), cobalt(II), nickel(II) and zinc(II) //Indian journal of chemistry section a-inorganic bio-inorganic physical theoretical & analytical chemistry. – 1983. – Т. 22. – №. 7. – С. 568-571.
- Patel K.S., Odunola O.A. Synthesis and physicochemical studies of some oxovanadium(IV) complexes of hydroxy-and aminobenzoates //Synthesis and Reactivity in Inorganic and Metal-organic Chemistry. 1990. V.20. №.6. P. 773-781.
