Introduction: Tolvaptan is a vasopressin receptor antagonist primarily used to manage hyponatremia (low sodium levels in the blood) in certain clinical situations. It also plays a key role in slowing the progression of autosomal dominant polycystic kidney disease (ADPKD). Hyponatremia can occur due to various underlying conditions, such as heart failure, liver cirrhosis, and the syndrome of inappropriate antidiuretic hormone (SIADH). In these conditions, the body may retain excessive water, diluting the sodium concentration in the blood. ADPKD is a genetic disorder marked by the development of numerous cysts in the kidneys, leading to gradual kidney dysfunction. Tolvaptan addresses these conditions by targeting the mechanisms driving these health issues.
The action of tolvaptan is based on its ability to block the effects of vasopressin (also known as antidiuretic hormone, ADH). Under normal conditions, vasopressin signals the kidneys to retain water, helping to regulate fluid balance. However, in conditions like hyponatremia and ADPKD, excessive water retention can be harmful. Tolvaptan works by blocking vasopressin receptors, specifically the V2 receptors in the kidneys, which inhibits water reabsorption. This promotes the excretion of excess water without causing the loss of essential electrolytes like sodium. As a result, tolvaptan helps to raise sodium levels in cases of hyponatremia and reduce fluid retention. In ADPKD, this reduction in fluid retention is believed to slow the growth of cysts and the deterioration of kidney function.
Tolvaptan is a potent drug that requires careful monitoring and should only be used under the supervision of a healthcare provider with experience in managing these conditions. Monitoring serum sodium levels is essential during treatment, as rapid changes in sodium concentrations can lead to severe complications, such as osmotic demyelination syndrome, a potentially irreversible neurological disorder. Additionally, tolvaptan may interact with other medications, so it is important for patients to inform their healthcare providers about all drugs, supplements, and over-the-counter medications they are using. Due to its specific mechanism of action and potential risks, tolvaptan is typically reserved for cases of hyponatremia that do not respond to other treatments or when the patient is symptomatic. Similarly, its use in ADPKD is considered for specific patient groups and requires careful evaluation of the potential benefits and risks.
Benefits of Developing and Validating Tolvaptan Analytical Methods
The development and validation of analytical methods for tolvaptan ensure that the drug meets quality standards and is free from impurities, which is vital for patient safety and therapeutic success. Analytical techniques such as High-Performance Liquid Chromatography (HPLC) and Ultra-High-Performance Liquid Chromatography (UHPLC) are commonly used to precisely measure tolvaptan in pharmaceutical formulations. These methods help ensure that the drug is administered at the correct dosage, maintaining therapeutic levels in the body, which is essential for treating conditions like hyponatremia and ADPKD.
The development and validation of these methods also allow for the optimization of the drug’s formulation. This includes improving the solubility, stability, and bioavailability of tolvaptan. For example, the creation of controlled-release formulations can enhance patient compliance by reducing the frequency of dosing. Validated analytical methods are essential for meeting regulatory standards set by agencies such as the FDA and EMA. These methods provide documented evidence that the drug is consistently produced and controlled according to stringent quality standards.
Furthermore, analytical methods are used in clinical trials to assess the pharmacokinetics, bioavailability, and potential interactions of tolvaptan with other medications. Post-market surveillance also relies on these methods to ensure the ongoing safety and efficacy of the drug in the general population.
Drug profile
Drug profile details of Tolvaptan
Structure
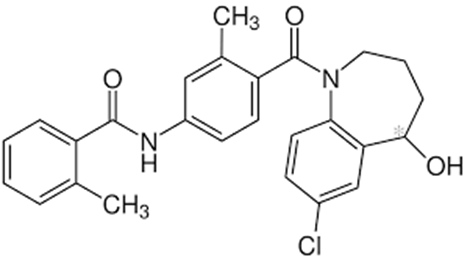
Figure 1: Structure of Tolvaptan [3]
IUPAC name : 4-[5-chloro-2-[[2-methyl-4-[(2-methylbenzoyl)amino]benzoyl]amino]phenyl]-4-hydroxybutanoic acid.
Introduction to Tolvaptan
Tolvaptan is a white, crystalline compound that forms prism-shaped crystals. Its molecular formula is C₂₆H₂₅ClN₂O₃, with a molecular weight of 448.94 g/mol. It has a melting point between 220°C and 226°C and a pKa of 7.4. Tolvaptan is practically insoluble in water, slightly soluble in ethanol, soluble in methanol, and readily dissolves in benzyl alcohol. It acts as a selective vasopressin V2-receptor antagonist, primarily used to slow kidney function decline in patients at risk of rapidly progressing autosomal dominant polycystic kidney disease (ADPKD). It is also effective in treating hypervolemic and euvolemic hyponatremia.
Drug Interactions
Tolvaptan can interact with various medications, potentially increasing side effects or reducing efficacy. Strong CYP3A inhibitors (e.g., ketoconazole, clarithromycin) can significantly increase tolvaptan plasma levels, raising the risk of adverse effects. Diuretics like furosemide may exacerbate dehydration and electrolyte imbalances. Additionally, antipsychotics and SSRIs may alter sodium and electrolyte levels. NSAIDs may impact renal function, and alcohol can worsen dehydration. Patients with hepatic or renal impairment should use tolvaptan with caution due to an increased risk of toxicity.
Mechanism of Action
Tolvaptan works by selectively and competitively blocking vasopressin V2 receptors in the renal collecting ducts. This inhibits vasopressin from triggering aquaporin-2 channel insertion into the apical membranes of duct cells, reducing water reabsorption. As a result, urine output increases, urine osmolality decreases, and serum sodium concentration rises—without significant loss of electrolytes. This aquaresis effect is particularly useful in correcting hyponatremia.
In ADPKD, blocking V2 receptors helps reduce cyst growth and fluid retention, slowing the decline in kidney function. Tolvaptan is approved for SIADH-related hyponatremia, as well as hyponatremia due to heart failure and liver cirrhosis, without causing major electrolyte imbalances.
Pharmacokinetic Properties
Absorption
Tolvaptan exhibits stereospecific pharmacokinetics, with a steady-state enantiomer ratio of approximately 3:1. While its absolute bioavailability is unknown, at least 40% of the administered dose is absorbed. Food does not significantly influence its absorption. Tmax and Cmax values differ between healthy individuals and those with heart failure.
Distribution
The drug has a high volume of distribution (~3 L/kg), indicating widespread tissue penetration. It is 99% plasma protein-bound, which limits its free concentration and tissue delivery.
Metabolism
Tolvaptan is mainly metabolized in the liver by CYP3A4, producing inactive metabolites such as DM-4103 and DM-4107. Its metabolism primarily involves hydroxylation and dehydrogenation.
Half-Life
It has an elimination half-life of around 12 hours, allowing for once-daily dosing and consistent therapeutic levels.
Excretion
Approximately 59% of the drug is eliminated via feces, with 40% excreted through urine, underscoring the role of hepatic metabolism in drug clearance.
Analytical Method Development and Validation
Chromatographic and Spectrophotometric Techniques
- Sruthi et al. (2017) developed a rapid, validated RP-HPLC method using sumatriptan as an internal standard and an Eclipse C18 column. The method was linear (5–100 μg/mL), with high accuracy (99–100.7%) and %RSD < 2.
- Srinivas et al. (2014) created a UV-spectrophotometric method using acetonitrile, with a λmax of 267 nm and linearity in the 1–10 μg/mL range. LOD and LOQ were 0.34 and 0.94 μg/mL, respectively.
- Eluri Samreen et al. (2024) validated an RP-HPLC method using a Hypersil BDS column, PDA detector at 254 nm, and an 8-minute runtime, meeting all ICH validation criteria.
- Shadab Anwar Hashmi et al. (2024) used QbD-assisted UHPLC, offering high accuracy (>99%), a 1.63 min retention time, and adherence to green analytical principles.
- Kumudini Rahul Pawar et al. (2023) established a sensitive Agilent HPLC-UV method suitable for bioanalytical applications, with a lower limit of quantification of 0.5 μg/mL.
- Vijaya Sri et al. (2014) confirmed UV-spectrophotometric method accuracy and precision under ICH guidelines.
- Sumera Iram et al. (2024) developed a reverse-phase HPLC method with UV detection at 235 nm, with a retention time of 2.57 min, demonstrating good linearity and recovery.
- Patel Seema et al. (2021) used UV and RP-HPLC methods with Sunsil C18 columns for routine pharmaceutical testing.
- Sutar et al. (2021) employed Design of Experiment (DoE) for a stability-indicating RP-HPLC method with LOD of 1.0871 μg/mL and high recovery (99.88%).
Mass Spectrometry and Electrochemical Analysis
- Venkata Ramu Derangula et al. (2013) developed an LC-ESI-MS/MS method for tolvaptan quantification in human plasma, with a fast 2-minute runtime and a linear range of 0.05–501 ng/mL.
- Prinesh N. Patel et al. (2015) used UPLC-PDA and Q-TOF-MS/MS to identify degradation products under forced degradation studies.
- Kohei Hoshikawa et al. (2019) developed an LC-MS/MS method for tolvaptan and its metabolites in human plasma.
- S. Rzeppa et al. (2016) created an HPLC-MS/MS method for detecting tolvaptan and its metabolites in urine, aiding anti-doping efforts.
- Umar J. Pandit et al. (2016) analyzed tolvaptan’s electrochemical behavior, revealing an irreversible oxidation process with nanomolar detection limits.
- Masayuki Furukawa et al. (2014) validated a method for tolvaptan and its metabolites in rat serum, used to assess sex-based pharmacokinetic differences.
Novel Delivery Systems
- Jong-Hwa Lee et al. (2022) optimized self-microemulsifying drug delivery systems (SMEDDS) using a Quality by Design (QbD) approach. Formulations showed significantly improved bioavailability (up to 33-fold higher) than raw tolvaptan.
Conclusion
Tolvaptan is an orally administered drug used for the treatment of hyponatremia caused by conditions such as SIADH and ADPKD. It works by blocking vasopressin V2 receptors, enhancing free water excretion (aquaresis) while conserving electrolytes. This mechanism helps correct water imbalances and slows kidney function decline in ADPKD patients, potentially reducing the need for dialysis or transplantation.
Tolvaptan is also approved for treating hyponatremia related to heart failure and liver cirrhosis. Its molecular structure (C₂₆H₂₅ClN₂O₃) and poor water solubility influence its formulation and delivery. Due to the risk of drug interactions and liver metabolism dependency, it must be used cautiously, especially in patients with hepatic or renal impairment.
The drug’s pharmacokinetics—characterized by high plasma protein binding, hepatic metabolism, and a 12-hour half-life—support once-daily dosing. Extensive research supports its clinical benefits. A 2007 study highlighted its effectiveness in treating hyponatremia, while 2017 findings confirmed its role in slowing ADPKD progression.
Analytical method development and validation are crucial for ensuring drug safety, quality, and efficacy. Techniques like HPLC, UHPLC, UV-spectrophotometry, and mass spectrometry have been successfully applied for its quantification. These methods are essential for regulatory compliance, clinical monitoring, and improving the formulation, bioavailability, and environmental sustainability of tolvaptan-based therapies.
Overall, tolvaptan represents a significant advancement in the management of hyponatremia and ADPKD, supported by robust scientific evidence and innovative analytical methods.
References
1. Derangula, V. R., Pilli, N. R., Bhukya, B. R., Pulipati, C. R., Adireddy, V., & Ponneri, V. (2013). Bioanalysis of tolvaptan, a novel AVP‐V2 receptor antagonist in human plasma by a novel LC‐ESI‐MS/MS method: A pharmacokinetic application in healthy South Indian male subjects. Biomedical Chromatography,28 (3), 332–340.
2.Sri, K. V., Sruthi, S., & Srinivas, L. D. (2014). UV spectrophotometric method for the estimation of tolvaptan in bulk and pharmaceutical formulations. Asian Journal of Research in Chemistry , 7 (9), 1–5. Retrieved from
3. Furukawa, M., Miyata, K., Kawasome, C., Himeda, Y., Takeuchi, K., Koga, T., Hirao, Y., & Umehara, K. (2014). Liquid chromatography–tandem mass spectrometry method for determining tolvaptan and its nine metabolites in rat serum: Application to a pharmacokinetic study. Archives of Pharmacal Research , 37 (12), 1578–1587. [https://doi.org/10.1007/s12272-014-0411-5](https://doi.org/10.1007/s12272-014-0411-5)
4. Patel, P. N., Kumar, D. R., Gananadhamu, S., & Srinivas, R. (2015). Characterization of the stress degradation products of tolvaptan by UPLC-Q-TOF-MS/MS. RSC Advances , 5 (27), 21142–21152. [https://doi.org/10.1039/C4RA16644B](https://doi.org/10.1039/C4RA16644B)
5. Pandit, U. J., Khan, I., Wankar, S., Raj, K., & Limaye, S. (2015). Development of electrochemical method for determination of tolvaptan at MWCNT/CPE in pharmaceutical preparations and human biological fluids. Analytical Chemistry Letters , 5 (6), 338–350. [https://doi.org/10.1080/22297928.2015.1110626](https://doi.org/10.1080/22297928.2015.1110626)
6. Rzeppa, S., & Viet, L. N. (2016). Analysis of tolvaptan and its metabolites in sports drug testing by high‐performance liquid chromatography coupled to tandem mass spectrometry. Drug Testing and Analysis , 8 (10), 1090–1094. [https://doi.org/10.1002/dta.1922](https://doi.org/10.1002/dta.1922)
7. Sri, K. V., Sruthi, S., & Madhuri, M. A. (2017). Rapid RP-HPLC method development and validation of tolvaptan in bulk and pharmaceutical dosage form for an internal standard. Asian Journal of Pharmaceutical Analysis 7(1), 36–40. [https://doi.org/10.5958/2231-5675.2017.00007.2](https://doi.org/10.5958/2231-5675.2017.00007.2)
8. Hoshikawa, K., Naito, T., Saotome, M., Maekawa, Y., & Kawakami, J. (2019). Validated liquid chromatography–tandem mass spectrometry method for simultaneous quantitation of tolvaptan and its five major metabolites in human plasma. Annals of Clinical Biochemistry , 56 (3), 387–396.
