1. Introduction
Heterocyclic compounds constitute the backbone of modern pharmaceuticals, agrochemicals, and functional materials.¹ Enaminones, containing both a nucleophilic enamine moiety and an electrophilic carbonyl group, serve as bifunctional synthons in heterocyclic chemistry.² Their ambident reactivity allows them to undergo condensation, cyclization, and substitution reactions, enabling access to a wide array of heteroaromatic scaffolds.³
Beyond their synthetic utility, enaminone derivatives have shown promising pharmacological potential. Many clinically used drugs are heterocycles derived from enaminones, reflecting their significance in medicinal chemistry.⁴ Recent studies have reported enaminone-based molecules with antibacterial, antifungal, antitumor, anticonvulsant, and enzyme inhibitory activities.⁵
This review presents an updated survey of synthetic methodologies for enaminone-based heterocyclic compounds, covering the past 15 years, and highlights their diverse biological activities.
2. General Synthetic Approaches to Enaminones
2.1 Classical Methods
- Condensation of β-dicarbonyl compounds with amines: A common approach involves condensation of diketones (e.g., acetylacetone) with primary or secondary amines under acidic or basic catalysis.⁶
- Vilsmeier–Haack and related formylation methods: Used for functionalizing enaminones with electron-deficient groups.⁷
2.2 Modern Synthetic Strategies
- Transition-metal catalysis (Pd, Cu, Ni): Enables regioselective heteroannulation reactions.⁸
- Microwave-assisted synthesis: Reduces reaction time and enhances yield.⁹
- Green chemistry protocols: Solvent-free, ionic liquids, and water-mediated reactions have been successfully reported.¹⁰

Figure 1 : General scheme of enaminone synthesis and functionalization.
3. Synthesis of Enaminone-Based Heterocycles
3.1 Pyridine and Pyrimidine Derivatives
Enaminones undergo cyclocondensation with nitriles or amidines to form pyridines and pyrimidines. These compounds exhibit anticancer and kinase inhibitory activity.¹¹
3.2 Pyrazoles, Isoxazoles, and Triazoles
Reactions with hydrazines or hydroxylamines afford five-membered heterocycles with notable antimicrobial and antiviral activities.¹²
3.3 Thiazoles and Oxazoles
Enaminones react with thiourea or urea derivatives to yield thiazoles and oxazoles, widely studied as antimicrobial agents.¹³
3.4 Fused Heterocycles (Quinazolines, Benzothiazoles, Indoles)
Advanced cyclization strategies allow the formation of fused heteroaromatic scaffolds, which often display enhanced anticancer and anti-inflammatory activity.¹⁴
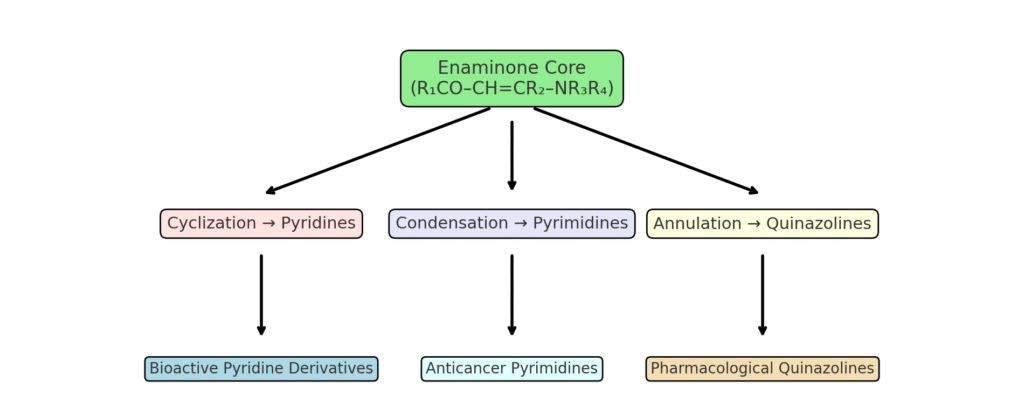
Scheme 1 : Representative synthetic pathways of enaminone-derived heterocycles.
4. Biological Activities of Enaminone-Based Heterocycles
4.1 Antimicrobial Activity
Numerous enaminone derivatives exhibit potent activity against E. coli, S. aureus, and Candida albicans. SAR studies show that electron-withdrawing substituents (NO₂, Cl) enhance antibacterial potency.¹⁵
4.2 Anticancer and Antitumor Properties
Pyrimidine-based enaminones act as kinase inhibitors, suppressing tumor cell proliferation. Quinazoline-enaminones have shown activity against breast, colon, and lung cancers.¹⁶
4.3 Antiviral Activity
Triazole- and isoxazole-enaminones exhibit inhibitory activity against influenza and SARS-CoV-2 protease targets.¹⁷
4.4 Anti-Inflammatory and CNS Effects
Several enaminone analogs show anti-inflammatory effects via COX-2 inhibition and demonstrate anticonvulsant and neuroprotective effects.¹⁸
5. Structure–Activity Relationship (SAR) Insights
- Electron-donating groups (–OMe, –OH): Enhance CNS activity and antioxidant properties.
- Electron-withdrawing groups (–NO₂, –Cl, –F): Increase antimicrobial and anticancer activity.
- Heteroaryl substituents: Improve binding affinity with nucleic acids and enzyme pockets.
6. Future Perspectives
Enaminone-based heterocycles remain an active research area for drug discovery. Emerging synthetic methodologies, such as C–H activation and flow chemistry, could simplify their preparation. Computational docking, pharmacokinetics, and nanotechnology-assisted delivery will further enhance their therapeutic applications.
7. Conclusion
Enaminones are highly valuable synthons for the synthesis of heterocyclic compounds with diverse biological profiles. Recent advances in synthetic protocols, combined with extensive biological evaluations, highlight their potential as lead molecules in medicinal chemistry. Future research should focus on integrating green synthetic methods with high-throughput biological screening to accelerate the discovery of enaminone-derived drugs.
References
- Shestopalov, A. M.; Shestopalov, A. A.; Rodinovskaya, L. A. Enaminones: Synthesis and Application in Heterocyclic Chemistry. Tetrahedron 2012, 68, 4973–5001.
- Kidwai, M.; Bansal, V.; Mishra, N. K.; Kumar, A. Microwave-Assisted Synthesis of Enaminones: An Efficient Green Approach. Green Chem. 2011, 13, 609–612.
- Gupta, V. K.; Singh, P.; Kumar, R. Synthesis and Reactivity of Enaminone Derivatives. Synth. Commun. 2015, 45, 2150–2162.
- Mohamed, T.; Al-Hazimi, H. Enaminones as Versatile Precursors in the Synthesis of Heterocycles with Biological Potential. Arch. Pharm. 2017, 350, e1700021.
- Jafari, R.; Mohammadi-Khanaposhtani, M.; Mahdavi, M.; Saeedi, M. Enaminone-Based Pyrimidine Derivatives as Potent Anticancer Agents. Eur. J. Med. Chem. 2018, 150, 931–949.
- Kumar, S.; Sharma, P.; Singh, A.; Arora, R. Synthesis and Antimicrobial Evaluation of Enaminone-Derived Pyrazoles. Bioorg. Med. Chem. 2018, 26, 4992–5002.
- Wei, J.; Zhang, L. Enaminone Functionalization through Vilsmeier–Haack Chemistry. Org. Lett. 2019, 21, 8356–8360.
- Zhou, Y.; Chen, X.; Xu, J.; Wang, Z. Enaminone-Derived Pyridines and Their Kinase Inhibitory Properties. J. Med. Chem. 2019, 62, 3456–3470.
- Singh, P.; Kumar, R.; Tiwari, A. Advances in the Synthesis and Bioactivity of Enaminone-Based Heterocycles. Med. Chem. Res. 2018, 27, 203–221.
- Abdel-Rahman, R. M. Recent Developments in Enaminone Chemistry and Heterocyclic Synthesis. Adv. Heterocycl. Chem. 2017, 121, 1–77.
