Introduction
Atherosclerosis is a complex and chronic disease involving gradual accumulation of lipids, collagen , elastic fiber , and proteoglycans in the arterial wall.
Pathogenesis of atherosclerosis is becoming better understood (1).Because of some studies showed clearly provides now free radical involvement in the pathogenesis of atherosclerosis itself (2). Hcy may promote atherogenesis through oxidative stress the mechanisms by which Hcypromotes atherosclerosis is not fully understood (3).Hcy is nonessential amino acid of molecular weight (268 dalton), formed during the metabolism of methionine (4)(5). Recently, in both clinical and experimental studies it has been discovered that a moderate increase of plasma Hcy level to confer an independent vascular disease risk factor (6)(7). It has also been revealed that hyperhomocysteinemia (HHcy) lead to the hurried process of atherosclerotic in diabetic patients (8). In this study, we aimed to evaluate the serum Hcy levels and serum MAD in patients with atherosclerosis disease and to find whether these variables are correlated with the severity of disease atherosclerosis disease.
Risk factors of Atherosclerosis
The complex process of atherosclerosis initiated and accelerated bythe role of important risk factors. One of the most important factors that play a role in the complex process of atherosclerosis is Oxidative stress and free radicals (9). Lipid peroxidation reactions begin after hydrogen atom is extracted from unsaturated fatty acid in the LDL particle by free radicals. Peroxidations of lipid are highly in polyunsaturated fatty acids, because the oxidation ability of fatty acid are increases with double bonds number. The main produced of the process of lipid peroxidation is MAD; carbonize group is used widely in determining oxidative stress (11). Cholesterol has a main impact in pathologic processes as a factor in the genesis of vital arteries atherosclerosis, causing , cerebrovascular, coronary and peripheral vascular disease. However, it is found as a chief constituent of several lipoproteins particles (12).
Material and Method
This study has been carried out at the consultative clinic in Baquba, for the period from September 2016 to May 2017. The study included (75) subjects (35 females and 40 males) with age range ( 59-72 ) years , (15) healthy individuals as control group and (60) patients(29 females and 31 males) with atherosclerosis. Patients were divided to three groups (Low risk= 1.0, moderate risk = 3.55, and high risk= > 6.15) according to the value of atherogenic index. Seven milliliters (mL) blood sample was collected into one 10 mL a plain tube from each participant after (12-14) hours fasting. Samples were stored at -20°C until biochemical assessment after separated by centrifugation at (3000 rpm) for (15 min) to collect serum.
Assessment of Atherosclerosis Risk factors
A height in cm and weight in kg were measured for all participant and BMI = weight / (height)2 by same investigator and tools was calculated. The enzymatic methods were used to estimate the following variables :triglyceride , total cholesterol (TC) concentrations. LDL-cholesterol concentrations were measured by using the Friedewald formula. Serum MDA is estimated by methods of Benge (1978) (13). Homocysteine were quantitatively determined in patients and control subjects by means of competitive ELISA test using commercially available kits. Atherogenic index is the ratio between total cholesterol / HDL- cholesterol(14). To calculate Atherogenic index the following equation was used (14): Atherogenic Index = TC / HDL- C. The dependent value of atherogenic index is as follows(: Low risk= (1.0) moderate risk = (3.55) high risk=> 6.15
Statistical analysis: The statistical tests used to analyze the data were as the following: Receiver operator curve (ROC) analysis was also employed. The area under the ROC curve gives an idea about the usefulness of a tested parameter in differentiating between two groups (one of which is a control group). In this context the ROC analysis helps in comparing selected parameters to others. The closer the area to one (ideal test) the more useful it is in discrimination. The Kruskal-Wallis test was used to analysis significance statistically of difference in atherogenic value index. Between more than two groups. ANOVA (f-test) was used to compare the three groups. Correlation coefficient (r), to find the correlation between two parameters within one group
Result
1.Receiver Operator Curve (ROC) Analysis
To discriminate between atherosclerotic patients and controls by employing the forthcoming investigated parameters, the ROC analysis was applied. Such analysis Allows to regulate the parameters according to the ROC area that can occupy and if such occupation is significant or not. The ROC analysis showed the descending order (Hcy = 0.959; MAD = 0.832; TC = 0.609; LDL = 0.594;and Tri = 0.502) of parameters that showed a significant variation. The rest of parameters (Uric Acid, Albumin and HDL-C) failed to occupy a significant ROC area (Table 1)
Table (1) Receiver operator curve (ROC) analysis for the investigated parameters in atherosclerotic patients and controls.
| Parameter | ROC Area | P ≤ |
| Hcy | 0.926 | 0.001 |
| MAD | 0.832 | 0.01 |
| TC | 0.609 | 0.01 |
| LDL-C | 0.594 | 0.05 |
| Tri | 0.502 | 0.05 |
| HDL-C | 0.402 | (N.S) |
| Uric Acid, | 0.452 | (N.S) |
| Albumin | 0.372 | (N.S) |
N.S.: Not significant (P > 0.05).
2.Distribution of patients according to value of atherogenic index.
We notice that there have been a highly significant differences (P<0.01) in value of atherogenic index. (73.3 %) of patients have high risk= > 6.15, while (15%) of the patients are considered to be moderate risk having 3.55 and (11.6%) of them are considered to be goals for atherogenic index in patients with atherosclerosis was low risk =1as shown in table 2
Table (2) Distribution of patients according to value of atherogenic index.
| value of atherogenic index | Gender | N | % | Comparison of Significance by Kruskal-Wallis Test |
| Lowrisk= 1.0 | Males | 3 | 11.6 | P<0.01 |
| females | 4 | |||
| moderate risk = 3.55 | Males | 3 | 15 | |
| Females | 6 | |||
| high risk= > 6.1 | Males | 25 | 73.3 | |
| Females | 19 | |||
| Total | 60 | 100 | ||
Significant at 0.01 level of significance using Kruskal-Wallis Test test for difference of more than two means
3.Investigated ParametersImpact on Atherogenic index
To investigate the impact of investigated parameters (Hcy and oxidative stress, as defined by MAD, on the Atherosclerosis, statistical differences between means of these parameters were assessed among the three groups of atherogenic index; Low risk= 1.0, moderate risk = 3.55, and high risk= > 6.15). Also correlation coefficient (r)assessed between these parameters and value of atherogenic index
3.1 Serum homocystein (Hcy) and correlation with atherogenic index
Table 3 and Figure (1 and 2)Showed the serum Hcy a gradual increased level in Atherosclerosis patients (2.26± 0.21 ,4.09± 0.25, and 8.45±0.16mg/mL, respectively) in the three progressed groups of Atherosclerosis (low, moderate and high risk). Such differences were significant in patients (P ≤ 0.05), moreover the r value (0.822) was also significant (P ≤ 0.001) in patients.
Table 3- : homocystein serum level in Atherosclerosis patients defined by the value atherogenic index
| Subjects | Serum Level of Hcy (mg/mL) | atherogenic index | ||
| Low risk= 1.0 | moderate risk = 3.55 | high risk= >6.15 | ||
| Patients | Number | 7 | 9 | 44 |
| Range | 1.8-5.03 | 1.9-6.21 | 4.05-10.07 | |
| Mean± SE | 2.26± 0.21 | 4.09± 0.25 | 8.45±0.16 | |
| ANOVA Probability < 0.05 (significant) | ||||
| r =-0.785 ; P < 0.001 (significant) | ||||

Significant at 0.05 level of significance using ANOVA test for difference of more than two means
Figure 1: Serum homocystein level in Atherosclerosis patients defined by the value atherogenic index

Figure 2: Correlations of Hcy and the value atherogenic index in patients groups
3.2 SerumMADand correlation with atherogenic index
The patients group demonstrated a gradual increase of MAD level (0.32 ± 0.12 0.65± 0.19M and 1.09±0.23μ mol / L, respectively) in the three groups of Atherosclerosis. Such difference was significant (P ≤ 0.05), and the r value (0.59) of correlation was also positively significant (P ≤ 0.01) as shown in table 4 and figure (3 and 4)
Table 4- : MAD serum level in Atherosclerosis patients defined by the value atherogenic index
| Subjects | Serum Level of MAD(μ mol / L) | atherogenic index | ||
| Low risk= 1.0 | moderate risk = 3.55 | high risk= >6.15 | ||
| Patients | Number | 7 | 9 | 44 |
| Range | 0.2-0.31 | 0.4-0.67 | 0.81-1.23 | |
| Mean± SE | 0.32 ± 0.12 | 0.65± 0.19 | 1.09±0.23 | |
| ANOVA Probability < 0.05 (significant) | ||||
| r =-0.59 ; P < 0.001 (significant) | ||||
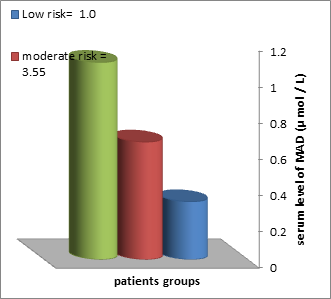
Significant at 0.05 level of significance using ANOVA test for difference of more than two means
Figure 3:MAD serum level in Atherosclerosis patients defined by the value atherogenic index

Figure 4: Correlations of MAD and the value atherogenic index in patients groups
Discussion
In the current study homocysteine increased significantly among atherosclerotic patients when compared to healthy control subjects by use ROC analysis as shown in table (1). Over the past three decades, homocysteine has relieved as uncommon risk independent marker for the procession of CVD (15). Baushey et al. (1995) have found a linear relationship between vascular risk and Hcy levels, that a 5 µmol/L increase in Hcy concentration was correlating with an increase in risk of vascular about thirds one (16). The relation between CVD and Hcy had investigated by several predictable studies. Many (17)(18) but not whole (19)(20) get it a positive relation .None of the preceding studies, however, studied the possibility of association between oxidative stress and Hcy with respecting to risk of Atherosclerosis. The strength relation between oxidative stress and hyperhomocysteinemia to be stronger among those withAtherosclerosis. Increasing oxidative stress by high concentrations of Hcy may exert an atherothrombotic effect, which may prompt dysfunction of endothelial. The results of the current study propose that serum Hcy is a predictor of atherosclerosis. However, levels of Hcy are correlated with value of atherogenicindex . Earlier studies on the serum level of Hcy as a predictor of atherosclerosis are argumentative. Clark et al.(1991), One main property of total Hcy(tHcy) is might to prompt atherogenesis Hcy reason atherogenesis by increasing DNA synthesis of a gene called Cyclin A which in turn prompt replication of an uncontrollable cells in one region within the blood vessels lining. In addition, tHcy may induce atherosclerosis by disruption the vasomotor endothelium regulation of nitric oxide by the forming of S-nitroso-homocysteine during a nitration process (21). Prasad (1999), Hcy has been supposed to induce atherosclerosis by the inducement the endothelium of oxidative injury, which is a crucial step in the restraint to injury hypothesis(22).Jakubowski et al. (2002) showed the important role of Hcy and oxidative – redox stress is biologically plausible because Hcy induce oxidant injury to vascular cells through the auto-oxidation of Hcy, forming of Hcy mixed disulfides, interaction of Hcythiolactones, and protein homocysteinylation, also forming of mixed disulfides contributes to the additional forming of ROS (23) Finally, harmony to Melvin et al. (2004) demonstrated increasing of serum Hcy in atheroscleropathy and concluded that homocysteinemia being an independent risk factor for CVD as happens in ischemic disease such as myocardial infarction and stroke and thrombotic events (24) .
MAD level was found to be higher in patients compared with the control groups (P > 0.01) as shown in table (1). These results are agreement to the some pervious study (25) (26) (27). Peroxidation of lipid produced MDA which is used widely in estimating oxidative stress therefore; the evaluation of MDA may be used to decide whether a process of lipid peroxidation has taken place (25). The measurement of MDA has widely been used to detect oxygen free radicals-mediated cell injury. In patients with atherosclerotic disease lipid peroxidation activity is accelerated. So higher MDA level is associated with the clean out of antioxidant and several forms of scavengers. It can be suggested that higher MDA levels might be a biochemical marker for atherosclerotic disease (27).
There is no doubt about the relation between serum lipids profile and the risk of chronic disease including CVD. As well as the metabolic syndrome, which is characterized by obesity, hypertension, glucose intolerance, visceral, and dyslipidemia (i.e. high serum TG and low serum HDL-C), that increases the risk for CVD. Our investigation agrees with Laaksonen et al. (2002)(28).The propagation of atherosclerosis a gradual increase with age. The aging process may promote modification of lipoprotein metabolic, which lead to an increase in the receptivity oxidation of the LDL-C because of their small size. So it is supposed that LDL are associated with increased atherogenesis(29). HDL-C is believed to act as cholesterol scavenger. It removes cholesterol first by absorption on its surface, and then facilitates enzymatic conversion to cholesterol esters which moves to the core of the HDL particle and returns to the liver. This property of removing cholesterol from peripheral tissues is thought to give HDL its favorable cardioprotective properties (30)(31).So for each 1mg/dL decrease in concentration of HDL-C , the risk for atherogenesis is increased by 2-3%in patients with low levels of HDL-C.
2.Distribution of patients according to value of atherogenic index.
We showed a highly significant differences in value of atherogenic index. Most of patients have high risk= > 6.15, while remind of the patients are considered to be moderate and low risk .unexpectedly high prevalence of coronary atherosclerosis in Iraqi people, atherosclerosis disease is more prevalent in males than females, and it is increasing with age progression, and the prevalence becoming 100% after the age of 40 years old (32). In addition, in population an aging that is at larger risk of developing these mortal and morbid CVD associated with diabetic, metabolic syndrome, and atheroscleropathy. To shorthand the risk of atheroscelerosis they should a devoted consideration for supplementation of folate and possible cobalamin, (24).
3.Hcy and MAD Impact on Atherogenic index
3.1 Serum homocystein (Hcy) and correlation with atherogenic index
The level of serum Hcy a gradual increased level in the third progressed groups of Atherosclerosis (low, moderate and high risk). Such differences were significant in patients; moreover the r value was also significant in patients. These results are similar to the observations of a number of other investigators (33) (34).Misra et al.(1994) relived that Hcy thiolactone , which forming during complicated rearrangements of Hcy is chemically reactive and acylates free amino groups in protein such as the side-chain lysine groups . In the process of homocysteinylated proteins formation moreover development oxidative stress and homocysteinylated proteins become damaged and may lose their biological activity. This lead to the alteration of proteins and in particular the alteration of LDL-C, which allow to their detention within the intima and following formation of inflammatory foam cell linked with atherogenesis. Jones et al. (1994), confirmed that oxygen free radicals generated from Hcy by noticing Hcy toxic effects and in the presence of Cu2+, and their association with increased peroxidation of lipid, which was inhibited by catalase and attenuated by desferal(35). Oxygen radicals have been confirmed to reason injury of endothelial. The oxidative hypothesis of atherosclerosis is based on oxidative stress prompt endothelial injury (36)(37)
3.2 Serum MADand correlation with atherogenic index
The patients group demonstrated a gradual increase of MADlevel in the thrid groups of Atherosclerosis. Such difference was significant, and the r value of correlation was also positively significant as shown in table 4. There are several previous studies that have studied the relation between MAD and aherosclerosis disease.Kostner et al. revealed higher levels of MDA in patients with coronary artery (38). Pucheu et al. showed an increase in level of serum MDA following thrombolysis in acute MI patients, but no significant differences they could find between control group and stable angina pectoris patients group (39). In (2001) Cavalca et al, in their study about oxidative stress and Hcy in coronary artery disease, he observed, higher significant levels of MDA were estimated in patients with coronary artery when compared to the healthy group (40).In our study, we found serum MAD was significantly increased in patients than control; it was gradually increased with increase atherogenic index, affected by the disease severity , and significantly increases in third group than first group of patients. We believed that MDA possibly also be associated with Atherosclerosis involvement.
Conclusion and Suggestions for future studies
In summary, there is a significant relation between Hcy levels, MDA, and atherosclerosis diseases. These conclusions promote the hypothesis that the opposing effect of oxidative stress on the atherosclerosis diseases might be mediated by Hcy. This relationship could cause early development of atherosclerosis diseases even in males. So we suggest that Hcy and MAD might be taken into consideration through the evaluation of atherosclerosis patients. In consistency of the results may relate to the fact that atherosclerosis is a very complex multiform disease, and the study population may therefore be very heterogeneous, most evidently. Further studies are required to assess the effect of nutrients supplementation such as folic acid, vitamin B6 (coblamine) &B12 which related directly to homocysteine metabolism in atherosclerotic patients. Extending the period of nutrients supplementation from three months to one year to show a better follow-up.Also further studies are needed to find the importance mineral such as (Zn,Ge, Se,Pb, Cr & Mg) and Xanthine oxidizes activity (NO synthetase) related with homocysteine metabolism in atherosclerotic patients.
Acknowledgment
My genuine thank to all patients and healthy subjects for their participation in this study. I wish to them along with best health. I would like also to thank the staff of biochemistry lab in the consultative clinic in Baquba. Also, my thanks go to any person who has given help and not mentioned.
Reference
- Thomas, M., Devling, Ph.D. BIOCHEMISTRY with clinical correlations, PUBLISHING USA 1982,9, 514 – 515.
- Steinberg, D and Witztum ,J,L. 1990. lipoproteins and Atherogenesis .current concepts, Jama 23: 264 -3047.
- Lawrence, M. P ; Title, M, D,; Peter, M. Cummings, B;,A, MSC, Karen Giddens, Jacques J; Genest, Jr, MD,; Bassam, A;Nassar, MB, BCH.. Effect of Folic Acid and Antioxidant Vitamins on Endothelial Dysfunction in Patients With Coronary Artery Disease, JACC , 2000,36( 3),758–65
- Tucker, K., L;Selhub, J; Wilson, P.,W; Rosenberg, I.,H. . Dietary intake pattern relates to plasma folate and homocysteine concentrations in the Framingham Heart Study. J. Nutr,1996 ,126,3025-31
- George., N.; Welcl, J.; L . New England. J. Medicine,1998,338(15),1042-50
- Boushey., C.,J;Beresford, S.,A.,A; Omenn, G.,S; Motulsky., A,G. . A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. JAMA, 1995,274, 1049-1057,
- Nygard., O.; Vollset., S,E; Refsum., H; Stensvold., I; Tverdal., A;Nordrehaug., JE;Ueland .,PM; Kvale., G. .Total plasma homocysteine and cardiovascular risk profile. JAMA ,1995,274, 1526-1533,.
- Okada., E; Oida., K; Tada., H; Asazuma., K; Eguch.,i K; Tohda., G; Kosaka., S;Takahashi., S; Miyamori., I. Hyperhomocysteinemia is a risk factor for coronary arteriosclerosis in Japanese patients with type 2 diabetes mellitus. Diabetes Care, 1999,22,484-490,
- Holvoe., P. . Oxidative modification of lowdensity lipoproteins in atherothrombosis. Acta Cardiol,1998,53,253–60
- Peter., NA; Caldwell., SE; and Mills., kA. Lipids mechanism of free radical oxidation of unsaturated lipids. McGraw-Hill, New York.1995,30: 277.
- Furchgott, RF and Zawadzki., JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature .1980,288,373-6
- Robert., K, Murray.; Daryl., K; Granner. (Eds). Harpers Illustrated Biochemistry.26th. McGrow-Hill Companies, United States. 2003,Ch.26,pp,219-20
- Benge J.A. and Aust S.D. Estimation of serum Malondialdehyde level in hoffee P.A .and Jones M.E. (eds), Methods in Enzymology Hoffee Jones. Academic Press, New York, San Francisco, London, ASubsidinary of Harcoart Brace Jovanovich, Publisher,1978,51,302.
- Wilson., P, W, F; Ordovas., J, M; and Namara., J, R, M. . Clinical chemistry: remnant lipoprotein cholesterol and triglyceride reference range from the Framingham heart study. Blackwell-Scientific Publication, London. 1998,44, 1224- 1232. .
- Rutishauser, W and Lerch, R. Asymptomatic ischemia, an important part of the spectrum of coronary disease; Schweiz. Rundsch. Med Prax. 1995 ,.84(42),1181-5
- Boushey.,CJ,;Beresford., SA; Omenn., GS; Motulsky., AG..Jama 1995,274,1049-57 .
- Stampfer., MJ; Malinow., MR; Willett., WC; Newcomer., LM; Upson., B; Ullmann., D; Tishler., PV;Hennekens., CH.. A prospective study of plasma homocysteine and risk of myocardial infarction in US physicians. JAMA. 1992,268,877-881.
- Verhoef., P; Hennekens., CH; Malinow., MR; Kok., FJ; Willett., WC;Stampfer MJ. A prospective study of plasma homocysteine and risk of ischemic stroke, 1994,25,1924-30.
- Evans., RW;Shaten., J; Hempel., JD; Cutler., JA ;Kuller., LH. For the MRFIT Research group. Homocystiene and risk of cardiovascular disease in the multiple risk factor intervention trial. ArteriosclerThrombVasc Biol. 1997 , 17,1947-53
- Alfthan., G; Pekkanen., J; Jauhiainen., M; Pitkaniemi., J; Karvonen., M, Tuomilehto., J; Salonen., JT ;Ehnholm., C.. Relation of serum homocysteine and lipoprotein concentrations to atherosclerotic disease in a prospective Finnish population based study. Atherosclerosis,1994 106,9-19.[Medline]
- Clarke., R, Daly.;R, RobinsonK, . Blood levels of homocystein and increased risks of cardiovascular diseases. N Engl J Med , 1991, 324:1149
- Prasad, K..Homocystein , a risk factor for cardiovascular diseases. Int J Angiol., 1999.,8:76
- Jakubowski, H..Homocysteine is a protein amino acid in humans. Implications for homocysteine-linked disease. J. Biol Chem,2002,277,30425–48.
- Melvin., R , Hayden and Suresh., C, Tyagi . Homocysteine and reactive oxygen species in metabolic syndrome, type 2 diabetes mellitus, and atheroscleropathy: The pleiotropic effects of folate supplementation. J. Nutr. 2004, 3:4.
- Bragt., PC, Bansberg., JI., and Bonta., IL.Anti-inflammatory effects of free radicals scavengers and antioxidants. Inflammation,1980,4, 289-299.
- Fukunaga., K;Takama., K and Suzuki., T. High-performance liquid chromatographic determination of plasma MDA level without a solvent extraction procedure. Anal Biochem,1995, 230, 20-23
- May, K Ismail. Ph.D .Thesis submitted to college of Science, University of Mosul Cupta S. and D eshmurk U.U. (1994 ) formation and function of free radicals in human body . Ann. Nat. Acad. Med. Science ,2006,30,45 – 59.
- Laaksonen., D, E; Niskanen., L; Punnonen., K; Nyyssonen., K;Salonen., R;Rauramaa., R. ; Salonen., JT. Sex hormone, inflammation and the metabolic syndrome: a population based study. European J. of endocrinol., 2003,149,601- 608.
- Khalil., A; Fortin., J; LeHoux., Jand Fulop., T. Age- related decrease of dehydroeepiandrosterone concentration in low density lipoproteins and its role in the susceptibility of low density lipoproteins to lipid peroxidation. J. Lipid Res., 2000,41, 1552- 1561.
- Zhao., X; Mrse., J. S; and Adowdy .,A. Safety and Tolerability of simvastatin plus niacin in patients with coronary artery disease and low high –density lipoprotein cholesterol. Am. G. Cardiol., 2004,93,307, 312.
- Davidsons., B. Principle and Practice of Medicine. 19th ed ., International Editor , Toronto , 2002,PP 420 – 437 .
- Ammar Mohammad Khudeer Al-Aamood ,Nemah H. Al-juboriandAli Al-Tmeme. Prevalence of Coronary Atherosclerosis in Babylon Governorate; Histopathological, Postmortem Prospective Study. Medical Journal of Babylon2015,Vol,12- No. 2
- Misra, HP. Generation of superoxide free radical during the autoxidation of thiols. J. Biol Chem, 1994,249,2151–2155
- Blom., HJ. Consequences of homocysteine export and oxidation in the vascular system. SeminThrombHemost. ,2000, 26,227–32
- Jones., BG; Rose., FA; Tudball., N. Lipid perioxidation and homocysteine induced toxicity. Atherosclerosis,1994,105:165.
- Weis., SJ; Young., J;LoBuglio., AF. Role of hydrogen peroxide in neutrophilmediated destruction of cultured endothelial cells. J Clin Invest. 1981,68:714.
- Kapoor, R and Prasad., K. Role of oxyradicals in cardiovascular depression and cellular injury in hemorrhagic shock and re-infusion: effect of SOD and catalase. Circ Shock,1994,43:79.
- Kostne.,r K; Hornykewyc.,z S;Yang., P. Is oxidative stress causally linked to unstable angina pectoris? A study in 100 CAD patients and matched controls. Cardiovasc Res, 1997 ,36,330–6 8.
- Pucheu., S;Coundray., C; Vanzetto., G; Favier., A, De; Liris., J. Assessment of radical activity during the acute phase of myocardial infarction following fibrinolysis: utility of assaying plasma malondialdehyde. Free RadicBiol Med,1995, 19,873–81 9.
- Cavalca., V; Cighett.,i G; Bamonti., F. Oxidative stress and homocysteine in coronary artery disease. Clin Chem, 2001,47(5),887-92
