- INTRODUCTION
Materials in nano-confines (1–100 nm) have remarkable differences in parcels compared to bulk materials. These differences exist in the physical and structural parcels of particles, molecules, and bulk paraphernalia owing to differences in physicochemical parcels and face-to-volume rates¹. With advances in nanotechnology, numerous nanomaterials with unique properties have emerged, opening new avenues for research. Metal nanoparticles, including silver, platinum, and gold, are used for various purposes2,3. AgNPs have been used extensively because of their unique physical and chemical characteristics, including electrical conductivity, thermal, optical, antimicrobial, and natural properties. AgNPs are widely used in biomedical applications, such as in crack dressings, antiseptic fabrics, creams, and sprays. AgNPs affect microorganisms through membrane disruption and enzymatic inhibition. Researchers have developed nanomaterials with distinct size-controlled physicochemical features and applications6. AgNPs are significant for their operations in healthcare, medicine, photothermal treatments, visual operations, catalytic processes, energy transfer, solar cells, optoelectronics, and environmental applications.7 Biological methods are safe, provident, doable, and ecologically sound, with downsides of time consumption and lower influence on size, shape, and liquid structure8. Among metallic rudiments, Ag is a noble metal that is central to antibacterial research. This review emphasizes the outlook, development, antibacterial functioning & mechanism, characterization methods, factors affecting bactericidal functioning, antimicrobial relevance, and future perspectives for green nanotechnology-based AgNPs in antibacterial therapeutic applications and infection control for SDG-39.
- THEORETICAL FRAMEWORK
Silver nanoparticles (AgNPs) have attracted attention from researchers because of their defense against microorganisms and drug resistance against commonly used antibiotics10. The characteristics of AgNPs have made them applicable in biomedical, drug delivery, water treatment, and agrarian fields11. AgNPs are used in ink bonds, electronic devices, and pastes because of their high conductivity12. AgNPs have been synthesized using various physicochemical methods, such as chemical reduction, gamma shaft radiation13, microemulsion, electrochemical system14, laser ablation15, autoclave, microwave oven roasting, and photochemical reduction. These methods have effective yields but are limited by toxic chemicals, high costs, and energy requirements. Given these downsides, cost-effective and energy-efficient approaches for AgNP emulsions using microorganisms, plant extracts, and natural polymers as reducing and capping agents are emerging. The combination of nanotechnology and green chemistry will expand biologically and cytologically compatible metallic nanoparticles16,17,18.
Phytochemicals in plant leaves, including flavonoids, alkaloids, ketones, carboxylic and ascorbic acids, tannins, amides, and phenols, drive the development of metal nanomaterials. These substances reduce metal salts to produce metal nanoparticles19 and exhibit antibacterial and antioxidant properties. Green synthesis of nanoparticles has lower toxicity and higher biocompatibility than chemical synthesis because of the longer nanomaterial half-life and increased efficiency20. Although the green synthesis of silver nanoparticles has been extensively studied, particularly regarding the synthesis using bacteria and fungi, to our knowledge, there is a lack of detailed information on environmentally friendly synthesis using plant extracts or their antiviral, antifungal, antimicrobial, and anticancer qualities21.
Table 1: Silver nanoparticles, their characterization, and applications
| S. No | Metal nanoparticles | Reducing agent or extract | Characterization | Particle characteristic | Applications | Author | References |
| 1 | AgNPs | Sambucus ebulus | FTIR, UV-Vis XRD HPLC | Size 18.6nm Shape- FCC | Antioxidant,& antibacterial activity | Karan T. et al. (2024) | [22] |
| 2 | AgNPs | Rubus discolor leave | UV- Vis, TEM FTIR EDX | Size-37 nm Shape- predominantly spherical | Antibacterial activity against Escherichia coli and Pseudomonas aeruginosa | Ghasemi S. et al. 2024) | [23] |
| 3 | AgNPs | Ocimum tenuiflorum and Azadirachta indica | UV- Vis XRD | Shape- FCC Size- 21 nm | S.aureus, E. coli, P.aeruginosa | Reen G. K.. et al. (2024) | [24] |
| 4 | AgNPs | Azadirachta indica, Syzygium aromaticum | UV-Vis, | Against Enterococcus | Chandran N. et al. (2024) | [25] | |
| 5 | AgNPs | Terminalia bellirica | UV- Vis, FTIR, XRD, FESEM | Size- 44.5 nm Shape- FCC | Antimalarial activity | Singh S. et al. (2024) | [26] |
| 6 | AgNPs | Pimpinella anisum aqueous seed extract | UV- Vis FTIR XRD | Shape- spherical Size – 20.18 nm, 21.00 nm, 40.08 nm | Antibacterial against a strain of Escherichia coli | Barabadi H. et al.(2023) | [27] |
| 7 | AgNPs | Moringa oleigera leaf extract | UV- Vis, FTIR, TEM | Size – 24 -40 nm | antibacterial Staphylococcus aureus ATCC6538 and pseudomonas aeruginosa ATCC9027 | Shaaban T. M. et al (2023) | [28] |
| 8 | AgNPs | Equisetum diffusum extract | UV- Vis FTIR, DLS, SEM EDX | Size- 62.6 nm | Antibacterial L.monocytogenes and E. coli | Jabbar A. et al (2023) | [29] |
| 9 | AuNPs | Gelidiella acerosa | UV-Vis FTIR | Size- 5-20 nm Shape- Spherical | Antibiotic against Staphylococcus aureus and, G. acerosa | Subbulakshmi A. et al (2023) | [30] |
| 10 | AgNPs | Green tea leaf extracts | UV- Vis., FTIR | Size- 30 – 150 nm Shape- quasi-spherical | High killing ability for bacteria (E. Coli) | Parvathalu K., et al (2023) | [31] |
| 11 | AgNPs | Eucalyptus camaldulensis and Terminalia arjuna extracts | UV- Vis, SPR, SEM , FTIR | Size- 23nm, 13 nm, 42 nm | Bacillus subtilis, Staphylococcus aureus, Pasteurella mutocida | Liaqat N. et al (2022) | [32] |
| 12 | AgNPs | Tridax procumbens plant (TNP) extracts | TEM, Zeta potential FTIR, HRLC- MS HPLC | Size- 23.17 nm Shape- FCC | Against Anticancer Escherichia coli, Shigella spp., Aeromonas spp. | Pungle R. et al.(2022) | [33] |
| 13 | AgNPs | Conocarpus Lancifolius fruit extracts | UV- Vis, FTIR, XRD | Size- 22.5 nm, Shape – spherical | Anticancer and Antibacterial Streptococcus p. , S. Aureus. | Oves M.et al. (2022) | [34] |
| 14 | AgNPs | Syzygium cumini fruit extract | UV- Vis, FTIR, XRD, SEM | Size- 47 nm Shape- FCC | Inhibit free radical orientation diseases., (G +ve & G –ve) bacteria | Chakravarty A. et al. (2022) | [35] |
| 15 | AgNPs | Cassia tora seed extracts | UV- Vis, FTIR, SEM, XRD | Size- 55.80 nm, 58 nm, 61.06 nm, 63.26 nm, 64.80 nm | Antibacterial efficacy against S. aureus., | Nawabjohn M. S. et al (2022) | [36] |
| 16 | AgNPs | East extract, malt extract | UV- Vis, FTIR, TEM, XRD | Size- 13.2 nm Shape – spherical | Antibacterial & cancer activity against (MCP) Escherichia coli, Klebsiella pneumonia | Wypij M. et al.(2021) | [37] |
| 17 | AgNPs | Cucumber leaf and rice husk extract | —– | ———– | Compared study between g-AgNPs & Chem. AgNPs Strong antibacterial activity Escherichia coli | Zhang H. et al (2021) | [38] |
| 18 | AgNPs | Areca catechu extract | UV- Vis, SEM, DLS, FTIR | Size – 25 nm Shape- spherical (round) | Against antibiotic-resistant bacteria | Choi S. J. et al (2021) | [39] |
| 19 | AgNPs | Berberis vulgaris B. nigra, Capsella bursa-pastoris, L. angustifolia plant extract | UV- Vis, FTIR, TEM, XRD, PCCS | Size- 46.1 nm Shape- spherical, truncated octahedron | Antibacterial activity L.monocytogenes (gram +ve) | Salayova A. et al. (2021) | [40] |
| 20. | AgNPs | Grapefruit peel extract | UV- Vis, FTIR | Size – 13.56 nm | Staphylococcus A., Enterococcus faecalis & S. aureus | Arsene M.M.J. et al (2021) | [41] |
| 21 | AgNPs | Onion (O), Tomato (T), Acacia catechu (C) alone, and Mixed COT extracts | UV- Vis, FTIR, TEM, XRD, DLS | Size – 25 nm Shape – FCC | Phytocatalitical Deradation of methyl orange (MO), methyl red (MR), & congo red (CO) | Chand K. et al. (2020) | [42] |
| 22 | AgNPs | Brillantaisia patula, Crossopreryx febrifuga, and Senna siamea leaf extract | UV- Vis, FTIR, XRD, TEM | Size- 17 nm Shape- spherical | (G +ve and G – ve Bect.) Staphylococcus A., Escherichia coli & Pseudomonas A. | Kambale K. E. et al. (2020) | [43] |
| 23 | AgNPs | Ocimum Canum Sims leaf extract | XRD, SEM | Size – 15.72 nm Shape- spherical and rod shape | Escherichia coli | Tailor G. et al (2020) | [44] |
| 24 | AgNPs | flower extract of Aerva lanata | UV- Vis, FTIR, AFM, SEM, TEM | Size- Shape – | Bacterial activity against Klebsiella planticola | Kanniah P. et al (2020) | [45] |
| 25 | AgNPs | Gymnema sylvestre leaf extract | UV- Vis, FTIR, XRD, TEM, | Size – 20 nm & 30 nm Shape – FCC | Staphylococcus aureus and Escherichia coli | Rajkumar P. V. et al (2020) | [46] |
The plant species used for the eco-friendly synthesis of AgNPs are listed in Table 1. Field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), and X-ray diffraction (XRD) are common methods for confirming nanoparticle formation and examining their shape, size, and surface area. The diameters of the reported particle sizes ranged from 5 to 100 nm, except for one. Their shapes included spherical, face-centered cubic (FCC), quasi-spherical, and occasionally truncated or rod-like forms. Nanoparticles exhibit biological and catalytic actions, such as antibacterial, antioxidant, anticancer, and photocatalytic effects, in addition to structural characterization. This indicates that Ag nanoparticles can be widely used in biomedical and environmental fields.
3. SYNTHESIS METHODS FOR NANOPARTICLES
3.1 Top-Down and Bottom-Up Methods: The synthesis of nanoparticles through biological systems is not only advantageous but also imperative for advancing sustainable technology. This method offers unparalleled benefits, including high-yield production, scalability, non-toxicity, and precise morphologies. It is crucial to pioneer novel methods for nanoparticle production, and green synthesis techniques are at the forefront of this innovation. These techniques are not only straightforward and safe but also environmentally friendly, making them indispensable in modern science47. Saratale et al have meticulously reviewed various green nanoparticle synthesis techniques, highlighting their transformative applications in agriculture and biomedicine48. The synthesis of green nanoparticles employs two distinct approaches: top-down and bottom-up approaches. The top-down approach involves mechanical methods to form larger nanoparticles, whereas the bottom-up approach, which is synonymous with green synthesis, utilizes acids to reduce the particle size. It is essential to integrate the bottom-up method with complex analysis when employing the top-down approach to maximize efficiency and effectiveness49. Embracing these green synthesis methods is not just a choice but a necessity for a sustainable future.
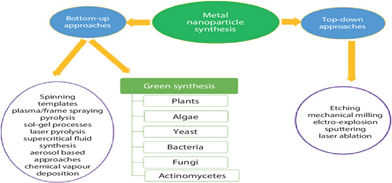
Figure 1: Top-down and bottom-up synthesis49.
- GREEN SYNTHESIS
Embracing the future of sustainable technology involves the environmentally friendly synthesis of AgNPs. By utilizing a solution of silver metal ions and an organic reducing agent, nanoparticle production can be revolutionized without the need for external stabilizing and capping agents. Remarkably, the natural components found in plants, such as flavonoids, alkaloids, ketones, aldehydes, amides, and ascorbic acid, serve as effective agents, making the process not only efficient but also eco-friendly. The synthesis of AgNPs is not just a possibility; it is a reality that harnesses the power of nature50.
The cornerstone of this innovative synthesis is Ag+ ions, which are readily available in various water-soluble silver salts. Among these, the aqueous AgNO3 solution stands out, with an optimal Ag+ ion concentration range of 0.1–10 nm, most commonly at 1 nm. This precise concentration ensured the successful development of AgNPs, paving the way for advancements in nanotechnology. By adopting this method, researchers can lead the charge in creating a sustainable future, demonstrating that cutting-edge technology and environmental responsibility can go hand in hand51.
3.3. AgNP SYNTHESIS USING PLANT EXTRACT
The green synthesis of AgNPs using plant extracts is not just an advancement in nanotechnology; it is a revolutionary leap forward, offering a sustainable and eco-friendly alternative to traditional methods of synthesis. Harnessing the power of various plant extracts from leaves, flowers, bark, roots, and stems, especially from medicinal plants like Azadirachta indica51, Aloe vera52, Ocimum tenuiflorum53, Emblica officinalis54, Tinospora cordifolia55, Cocos nucifera56, and common spices such as piper nigrum57, serves as a potent and natural solution. These plant components underwent a meticulous cleaning process, first with tap water and then with distilled water, to ensure their absolute purity. After drying, they were either ground into a fine powder or used directly to create extracts with varying pH values, while enhancing the stability of AgNPs, with protein metabolites and chlorophyll acting as effective agents58. The efficiency of this process is undeniable, as evidenced by the synthesis of spherical AgNPs averaging 20 nm in a mere 40 min using 60 ml of plant leaf extract and 10 ml of AgNO3 at 60°C. Prathinha et al.59 further substantiated this method by successfully synthesizing AgNPs using Azadirachta indica and Ocimum tenuiflorum green extracts. The striking visual transformation from green to dark brown is a definitive indicator of successful AgNP formation (Figure 2). Importantly, higher concentrations of plant extracts significantly accelerate the synthesis rates 25, while factors such as pH, temperature, optical activity, and AgNO3 concentration are crucial for optimizing production. The precise collection of AgNPs for characterization was ensured by centrifuging the silver nitrate and plant solutions24. Neem and Ocimum tenuiflorum extracts have been extensively studied, with neem plants exhibiting extraordinary antibacterial and antifungal properties, offering promising applications in bacterial treatment, as illustrated in Figure 3. This innovative approach to AgNP synthesis champions environmental sustainability and holds immense potential for propelling medical and technological advancements60,62.

Figure 2: Tulsi extract explanation setup during AgNP formation59.
This groundbreaking approach not only underscores the remarkable versatility of plant materials but also highlights their pivotal role in advancing nanotechnology. The evidence is clear: plant-based synthesis of AgNPs is not just a possibility but a revolutionary step forward in the field63.
4. ANTIMICROBIAL PROPERTIES OF THE PRODUCED AgNPs
4.1. Well Diffusion Assay
The antibacterial efficacy of AgNPs was rigorously evaluated using the agar well diffusion assay, a method that leaves no room for doubt. The results, measured by inhibition zones (mm) in 6 mm wells on Muller-Hinton agar, were nothing short of compelling. The particles, serially diluted 2-fold from 360 μL/mL to 11.25 μL/mL64, demonstrated a formidable antibacterial effect. E. faecalis, a notorious Gram-positive cocci bacterium, leading cause of enterococcal infections in humans and a prevalent pathogen in hospitals, often found in the urinary tract, bloodstream, and surgical sites. The positive control, vancomycin, exhibited its well-documented antibacterial action against E. faecalis, with an impressive inhibition zone of 27.0 ± 0.0 mm. However, the challenge of vancomycin-resistant enterococci (VRE)65, a global threat, remains unyielding, as no inhibition zone was observed for either VRE or E. faecalis strains. The AgNPs data, as illustrated in Figure 3, reveal a strain with sustained release, greater stability, tensile strength, nanoscale porosity, flexibility, and dominant antimicrobial efficacy against both gram-negative and gram-positive strains.
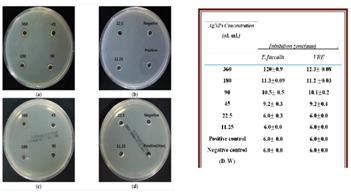
Figure 3: Antibacterial effects of AgNPs using A. catechu against E. faecalis and VRE. (a,b) Inhibition zone of AgNPs against E. faecalis; (c,d) inhibition zone of AgNPs against VRE62
This highlights the potential of microemulsions in wound healing applications66. P. aeruginosa, a gram-negative bacterium notorious for its green pigment pyocyanin, poses severe health risks, including meningitis, septicemia, post-transplant infections, persistent pneumonia, and urinary tract infections67. The rise of multidrug-resistant Pseudomonas aeruginosa (MRPA) is a serious public health concern, escalating morbidity and mortality in hospitalized patients. Alarmingly, MRPA showed no inhibition zone for gentamicin, whereas P. aeruginosa exhibited a substantial inhibition zone of 25.0 ± 0.7 mm diameter. Notably, both bacteria displayed concentration-dependent inhibition zones, with P. aeruginosa treated with AgNPs achieving a slightly larger inhibition zone than MRPA. The results, as depicted in Figure 4 and the accompanying Table, underscore the potential of AgNPs in combating these formidable pathogens65.
- CHARACTERIZATION:
The characterization of the synthesized AgNPs using techniques such as Fourier transform infrared spectroscopy (FTIR), transmission electron microscopy (TEM), ultraviolet-visible spectroscopy (UV-Vis), X-ray diffraction (XRD), and scanning electron microscopy (SEM) is critical for revealing details of size, shape, and surface charge 22,78. Dynamic light scattering (DLS) and zeta potential methods are essential for characterizing antibacterial agents, providing crucial insights into their application 42,66,79. Atomic force microscopy (AFM) and high-performance liquid chromatography (HPLC) provide additional information for characterizing AgNPs. The transformation of Ag+ to Ag° in the aqueous phase is evidenced by a band in the silver colloid spectra at approximately 410 nm33. As the silver concentration increased, the absorption band sharpened, and a red shift from 402 to 407 nm occurred (Figure 4) when the AgNO₃ concentration increased from 250 to 1000 mg/dm³, indicating larger silver aggregates and increased particle size. The TEM images confirmed the transformation of spherical silver particles into prismatic structures 67. The size, morphology, and surface charge of nanocarriers are pivotal determinants of their physical stability and function. Smaller particles with larger surface areas influence drug release, whereas the surface charge affects the interactions between the biological environment and bioactive compounds 70. The safety, behavior, and efficacy of biodistribution depend on these factors. A comprehensive description of AgNPs is essential to assess their functional elements.68
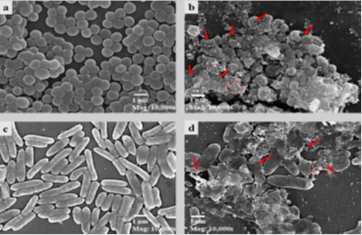
Figure 4: Scanning electron microscopy images of S. aureus (control and AgNP-treated) and P. aeruginosa (control and AgNP-treated). Red arrows indicate bacterial cell distortions, and the red circle shows nanoparticle accumulation on cells.62
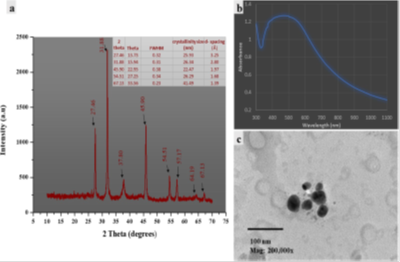
Figure 5: (a) XRD pattern showing peaks at 2-theta angles. (b) The curve shows the absorption feature, indicating the energy band gap. (c) TEM image reveals the spherical morphology and size distribution62.
6. APPLICATIONS OF GREEN SYNTHESIZED SILVER NANOPARTICLES FOR HEALTH AND WELL-BEING, SDG 3
Green-synthesized AgNPs have gained attention owing to their unique properties and applications across fields. Their environmentally friendly synthesis using biological agents offers advantages over traditional chemical methods of synthesis. AgNPs exhibit antimicrobial properties against bacteria, fungi, and viruses, making them promising candidates for use in wound dressings, medical devices, and water purification69,79. Green-synthesized AgNPs have applications in drug delivery, biosensing, and cancer therapy, enabling targeted drug delivery and cancer cell apoptosis70.
SDG 12 Catalysis: AgNPs are efficient catalysts in chemical reactions, including organic synthesis and environmental remediation. Their surface area and electronic properties enhance their catalytic activity71,78.
SDG 6, Environmental Remediation: Green-synthesized AgNPs remove pollutants from water and soil by adsorbing heavy metals and organic contaminants72,73,76,77.
Textile Industry: AgNPs incorporated into textiles provide antimicrobial properties, odor resistance, and UV protection, improving fabric hygiene and durability74,75.
7. FUTURE PROSPECTS OF GREEN SYNTHESIZED SILVER NANO-PARTICLES
Green-synthesized AgNPs represent a groundbreaking advancement with transformative potential in multiple industries, including personalized medicine, sustainable agriculture, and environmental remediation. These nanoparticles are not just an option; they are a necessity for achieving SDG-3 by promoting the good health and well-being of the population. In personalized medicine and tissue engineering, AgNPs offer unparalleled opportunities for innovation and improvements. As sustainable alternatives to chemical pesticides, they promise to revolutionize agriculture and ensure food security while protecting the planet. Moreover, their application in air purification systems and smart textiles for healthcare monitoring is expected to redefine industry standards. However, to fully harness these benefits, it is imperative to establish standardized protocols for their synthesis, application, and disposal. This will ensure safety and minimize environmental impacts. Furthermore, rigorous research on the long-term effects of AgNPs on human health and the environment is essential to determine safe usage levels. The time to act is now; embracing AgNPs is not just a choice but a strategic imperative for a sustainable future.
- CONCLUSION:
Characterization of green-synthesized AgNPs is imperative to unlock their potential in biomedical applications. Techniques such as SEM, TEM, UV-visible spectroscopy, FTIR, XRD, dynamic light scattering (DLS), and zeta potential measurements provide insights into the size, shape, morphology, and surface charge of these nanoparticles. The spectral shifts and morphological transformations from spherical to prismatic structures indicate changes in particle size and aggregation, which influence their functional properties, including drug release behavior and biodistribution. Advanced techniques such as atomic force microscopy (AFM), high-performance liquid chromatography (HPLC), and zeta potential measurements for antibacterial characterization highlight the role of surface charge in AgNP-biological interactions. Smaller particle sizes and optimal surface charges enhance AgNP efficacy by improving their interactions with bioactive compounds and refining their distribution. Therefore, a comprehensive characterization of AgNPs is essential for optimizing their application in antimicrobial treatments and ensuring their stability in biomedical contexts.
- ACKNOWLEDGMENT
I express my deep sense of gratitude to my principal, Professor S. P. Singh. St. John’s College, Dr. Bhimrao Ambedkar University, Agra, for their encouragement and constant help during the study period.
10. REFERENCES
- Vijayaram, S., Razafindralambo, H., Sun, Y. Z., Vasantharaj, S., Ghafarifarsani, H., Hoseinifar, S. H., & Raeeszadeh, M. (2024). Applications of green-synthesized metal nanoparticles: a review. Biological Trace Element Research, 202(1), 360-386
- Ranjan, S., Dasgupta, N., Chakraborty, A. R., Melvin Samuel, S., Ramalingam, C., Shanker, R., & Kumar, A. (2014). Nanoscience and nanotechnology in food industries: opportunities and research trends. Journal of nanoparticle research, 16, 1-23.
- Iravani, S., Korbekandi, H., Mirmohammadi, S. V., & Zolfaghari, B. (2014). Synthesis of silver nanoparticles: chemical, physical & biological methods. Research in Pharmaceutical Sciences, 9(6), 385-406.
- Pawar, A. A., Sahoo, J., Verma, A., Alswieleh, A. M., Lodh, A., Raut, R., … & Islam, M. R. (2022). Azadirachta indica‐Derived Silver Nanoparticle Synthesis and Its Antimicrobial Applications. Journal of Nanomaterials, 2022(1), 4251229.
- Bello, B. A., Khan, S. A., Khan, J. A., Syed, F. Q., Anwar, Y., & Khan, S. B. (2017). Antiproliferation and antibacterial effect of biosynthesized AgNps from leaves extract of Guiera senegalensis and its catalytic reduction on some persistent organic pollutants. Journal of Photochemistry and Photobiology B: Biology, 175, 99-108.
- Asif, M., Yasmin, R., Asif, R., Ambreen, A., Mustafa, M., & Umbreen, S. (2022). Green synthesis of silver nanoparticles (AgNPs), structural characterization, and their antibacterial potential. Dose-Response, 20(2), 15593258221088709
- Bindhu, M. R., Umadevi, M., Esmail, G. A., Al-Dhabi, N. A., & Arasu, M. V. (2020). Green synthesis and characterization of silver nanoparticles from Moringa oleifera flower and assessment of antimicrobial and sensing properties. Journal of Photochemistry and Photobiology B: Biology, 205, 111836.
- Vadakkan, K., & Rumjit, N. P., Ngangbam, A. K., Vijayanand, S., & Nedumpillil, N. K. (2024). Novel advancements in the sustainable green synthesis approach of silver nanoparticles (AgNPs) for antibacterial therapeutic applications. Coordination Chemistry Reviews, 499, 215528
- Dankovich, T.A. Gray, D.G. (2011) Bactericidal Paper Impregnated with Silver Nanoparticles for Point-of-Use Water Treatment. Environmental Science & Technology, 45, 1992-1998.
- Nair, R., Varghese, S.H., Nair. B.G., Maekawa. T., Yoshida, Y., and Sakthi Kumar, D. (2010) Nanoparticulate Material Delivery to Plants. Plant Science, 179, 154-163.
- Khan, Z., Al-Thabaiti, S.A., Obaid, A.Y., and Al-Youbi, A.O. (2011) Preparation and Characterization of Silver Nanoparticles by Chemical Reduction Method. Colloids and Surfaces B: Biointerfaces, 82, 513-517.
- Zhang W.Z., Qiao X.L., Chen J.G.. (2006). Synthesis and Characterization of Silver Nanoparticles in AOT Microemulsion System. Chemical Physics, 300, 495-500.
- Reicha, F.M., Sarhan, A., Abdel-Hamid, M.I., and El-Sherbiny, (2012) Preparation of Silver Nanoparticles in the Presence of Chitosan by Electrochemical Method. Carbohydrate Polymers, 89, 236-244.
- Song, J.Y. and Kim, B.S. (2009) Rapid Biological Synthesis of Silver Nanoparticles Using Plant Leaf Extracts. Bioprocess and Biosystems Engineering, 32, 79-84.
- Huang, H. and Yang, X. (2004) Synthesis of Polysaccharide-Stabilized Gold and Silver Nanoparticles: A Green Method. Carbohydrate Research, 339, 2627-2631.
- Philip, D. (2010) Honey Mediated Green Synthesis of Silver Nanoparticles. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 75, 1078-1081.
- Philip, D. (2010) Rapid Green Synthesis of Spherical Gold Nanoparticles Using Mangifera indica Leaf. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 77, 807-810.
- Bhardwaj, B., Singh, P., Kumar, A., Kumar, S., & Budhwar, V. (2020). Eco-friendly, greener synthesis of nanoparticles. Advanced Pharmaceutical Bulletin, 10(4), 566
- Nadaf, S. J., Jadhav, N. R., Naikwadi, H. S., Savekar, P. L., Sapkal, I. D., Kambli, M. M., & Desai, I. A. (2022). Green synthesis of gold and silver nanoparticles: Updates on research, patents & prospects. OpenNano, 8, 100076.
- Karan, T., Gonulalan, Z., Erenler, R., Kolemen, U., & Eminagaoglu, O. (2024). Green synthesis of silver nanoparticles using Sambucus ebulus leaves extract: characterization, quantitative analysis of bioactive molecules, antioxidant and antibacterial activities. Journal of Molecular Structure, 1296, 136836.
- Ghasemi, S., Dabirian, S., Kariminejad, F., Koohi, D. E., Nemattalab, M., Majidimoghadam, S. (2024). Process optimization for green synthesis of silver nanoparticles using Rubus discolor leaves extract and its biological activities against multidrug-resistant bacteria and cancer cells. Scientific Reports, 14(1), 4130.
- Reen, G. K., Sharma, P., & Kumar, A. (2024). Plant Extract-Mediated Synthesis and Antibacterial Potential of Metallic Nanoparticles. In Nanotechnology-Based Strategies for Combating Antimicrobial Resistance (pp. 117-152). Singapore: Springer Nature Singapore.
- Chandran, N., Ramesh, S., & Shanmugam, R. (2024). Synthesis of Silver Nanoparticles Using Azadirachta indica and Syzygium aromaticum Extract and Its Antibacterial Action Against Enterococcus faecalis: An In Vitro Study. Cureus, 16(7).
- Singh, S., Arya, H., Sahu, W., Reddy, K. S., Nimesh, S., Alotaibi, B. S. & Kumar Bhatt, T. (2024). Green synthesized silver nanoparticles of Terminalia bellirica leaves extract: synthesis, characterization, in silico studies, and antimalarial activity. Artificial Cells, Nanomedicine, and Biotechnology, 52(1), 238-249.
- Barabadi, H., Hosseini, O., Jounaki, K., Sadeghian-Abadi, S., Ashouri, F., Alrikabi, A. M. A., & Mostafavi, E. (2023). Bioinspired green-synthesized silver nanoparticles: in vitro physicochemical, antibacterial, biofilm-inhibitory, genotoxicity, antidiabetic, antioxidant, and anticoagulant performance. Materials Advances, 4(14), 3037-3054.
- Shaaban M. T., Zayed M., Salama H. S. (2023). Antibacterial potential of bacterial cellulose impregnated with green-synthesized silver nanoparticles against S. aureus and P. aeruginosa. Current Microbiology, 80(2), 75.
- Jabbar A, Abbas A, Assad N., Naeem-ul-Hassan M., Alhazmi H. A., Najmi, A., … & Amin, H. M. (2023). A highly selective Hg 2+ colorimetric sensor and antimicrobial agent based on green-synthesized Ag silver nanoparticles using Equisetum diffusum extract. RSC advances, 13(41), 28666-28675.
- Subbulakshmi, A., Durgadevi, S., Anitha, S., Govarthanan, M., Biruntha, M., Rameshthangam, P. (2023). Biogenic gold nanoparticles from Gelidiella acerosa: bactericidal and photocatalytic degradation of two commercial dyes. Applied Nanoscience, 13(6), 4033-4042.
- Parvathalu, K., Kumar, D. N., Rajitha, K., Kishan, M. G., Kumar, B. N., Bhemarajam, J., & Bhaskar, P. B. (2023). Facile synthesis of silver nanoparticles using green tea leaf extract and evolution of antibacterial activity. Plasmonics, 18(5), 1837-1845
- Liaqat, N., Jahan, N., Anwar, T., & Qureshi, H. (2022). Green synthesized silver nanoparticles: Optimization, characterization, antimicrobial activity, and cytotoxicity study by hemolysis assay. Frontiers in chemistry, 10, 952006.
- Pungle, R., Nile, S. H., Makwana, N., Singh, R., Singh, R. P., & Kharat, A. S. (2022). Green synthesis of silver nanoparticles using the Tridax Procumbens plant extract and screening of its antimicrobial and anticancer activities. Oxidative Medicine and Cellular Longevity, 2022(1), 9671594
- Oves, M., Rauf, M. A., Aslam, M., Qari, H. A., Sonbol, H., Ahmad, I., & Saeed, M. (2022). Green synthesis of silver nanoparticles by Conocarpus Lancifolius plant extract and their antimicrobial and anticancer activities. Saudi journal of biological sciences, 29(1), 460-471.
- Chakravarty, A., Ahmad, I., Singh, P., Sheikh, M. U. D., Aalam, G., Sagadevan, S., & Ikram, S. (2022). Green synthesis of silver nanoparticles using fruits extracts of Syzygium cumini and their bioactivity. Chemical Physics Letters, 795, 139493.
- Nawabjohn, M. S., Sivaprakasam, P., Anandasadagopan, S. K., Begum, A. A., & Pandurangan, A. K. (2022). Green synthesis and characterization of silver nanoparticles using Cassia tora seed extract and investigation of antibacterial potential. Applied Biochemistry and Biotechnology, 1-15.
- Wypij, M., Jędrzejewski, T., Trzcińska-Wencel, J., Ostrowski, M., Rai, M., & Golińska, P. (2021). Green synthesized silver nanoparticles: antibacterial and anticancer activities, biocompatibility, and analysis of surface-attached proteins. Frontiers in microbiology, 12, 632505.
- Zhang, H., Chen, S., Jia, X., Huang, Y., Ji, R., & Zhao, L. (2021). Comparison of the phytotoxicity between chemically and green synthesized silver nanoparticles. Science of the Total Environment, 752, 142264.
- Choi, J. S., Jung, H. C., Baek, Y. J., Kim, B. Y., Lee, M. W., Kim, H. D., & Kim, S. W. (2021). Antibacterial activity of green-synthesized silver nanoparticles using Areca catechu extract against antibiotic-resistant bacteria. Nanomaterials, 11(1), 205.
- Salayová, A., Bedlovičová, Z., Daneu, N., Baláž, M., Lukáčová Bujňáková, Z., Balážová, Ľ., & Tkáčiková, Ľ. (2021). Green synthesis of silver nanoparticles with antibacterial activity using various medicinal plant extracts: Morphology and antibacterial efficacy. Nanomaterials, 11(4), 1005.
- Arsène, M. M., Podoprigora, I. V., Davares, A. K., Razan, M., Das, M. S., & Senyagin, A. N. (2021). Antibacterial activity of grapefruit peel extracts and green-synthesized silver nanoparticles. Veterinary World, 14(5), 1330.
- Chand, K., Cao, D., Fouad, D. E., Shah, A. H., Dayo, A. Q., Zhu, K., … & Dong, S. (2020). Green synthesis, characterization and photocatalytic application of silver nanoparticles synthesized by various plant extracts. Arabian Journal of Chemistry, 13(11), 8248-8261
- Kambale, E. K., & Nkanga, C. I., Mutonkole, B. P. I., Bapolisi, A. M., Tassa, D. O., Liesse, J. M. I., & Memvanga, P. B. (2020). Green synthesis of antimicrobial silver nanoparticles using aqueous leaf extracts from three Congolese plant species (Brillantaisia patula, Crossopteryx febrifuga, and Senna siamea). Heliyon, 6(8).
- Tailor, G., Yadav, B. L., Chaudhary, J., Joshi, M., & Suvalka, C. (2020). Green synthesis of silver nanoparticles using Ocimum canum and their antibacterial activity. Biochemistry and Biophysics Reports, 24, 100848.
- Kanniah, P., Radhamani, J., Chelliah, P., Muthusamy, N., Joshua Jebasingh Sathiya Balasingh Thangapandi, E., Reeta Thangapandi, J., & Shanmugam, R. (2020). Green synthesis of multifaceted silver nanoparticles using the flower extract of Aerva lanata and evaluation of its biological and environmental applications. ChemistrySelect, 5(7), 2322-2331.
- Rajkumar, P. V., Prakasam, A., Rajeshkumar, S., Gomathi, M., Anbarasan, P. M., & Chandrasekaran, R. (2020). Green synthesis of silver nanoparticles using Gymnema sylvestre leaf extract and evaluation of its antibacterial activity. South African Journal of Chemical Engineering, 32(1), 1-4.
- Fardood, S. T., Ramazani, A., & Moradi, S. (2017). A novel green synthesis of nickel oxide nanoparticles using Arabic gum. Chemistry Journal of Moldova, 115-118.
- Saratale, R. G., Karuppusamy, I., Saratale, G. D., Pugazhendhi, A., Kumar, G., Park, Y., (2018). A comprehensive review of green nanomaterials using biological systems: Recent perception and future applications. Colloids and Surfaces B: Biointerfaces, 170, 20-35.
- Chokkareddy, R., Thondavada, N., Kabane, B., & Redhi, G. G. (2018). Current advances in biosynthesis of silver nanoparticles and their applications. Green metal nanoparticles: synthesis, characterization and their applications, 165-198
- Shankar, S. S., Ahmad, A., & Sastry, M. (2003). Geranium leaf-assisted biosynthesis of silver nanoparticles. Biotechnology progress, 19(6), 1627-1631.
- Tripathi, A., Chandrasekaran, N., Raichur, A. M., & Mukherjee, A. (2009). Antibacterial applications of silver nanoparticles synthesized by aqueous extract of Azadirachta indica (Neem) leaves. Journal of Biomedical Nanotechnology, 5(1), 93-98.
- Chandran, S. P., Chaudhary, M., Pasricha, R., Ahmad, A., (2006). Synthesis of gold nanotriangles and silver nanoparticles using Aloevera plant extract. Biotechnology progress, 22(2), 577-583.
- Patil, R. S., Kokate, M. R., & Kolekar, S. S. (2012). Bioinspired synthesis of highly stabilized silver nanoparticles using Ocimum tenuiflorum leaf extract and their antibacterial activity. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 91, 234-238.
- Ankamwar, B., Damle, C., Ahmad, A., & Sastry, M. (2005). Biosynthesis of gold and silver nanoparticles using Emblica officinalis fruit extract, their phase transfer and transmetallation in an organic solution. Journal of nanoscience and nanotechnology, 5(10), 1665-1671.
- Anuj, S. A., & Ishnava, K. B. (2013). Plant-mediated synthesis of silver nanoparticles by using dried stem powder of Tinospora cordifolia, its antibacterial activity, and comparison with antibiotics.
- Roopan, S. M., Madhumitha, G., Rahuman, A. A., Kamaraj, C., Bharathi, A., & Surendra, T. V. (2013). Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity. Industrial Crops and Products, 43, 631-635.
- Shukla, V. K., Singh, R. P., & Pandey, A. C. (2010). Black pepper-assisted biomimetic synthesis of silver nanoparticles. Journal of Alloys and Compounds, 507(1), L13-L16.
- Srikar, S. K., Giri, D. D., Pal, D. B., Mishra, P. K., & Upadhyay, S. N. (2016). Green synthesis of silver nanoparticles: a review. Green and Sustainable Chemistry, 6(01), 34.
- Prathibha, B. S., Harshitha, N., Neha, D. R., Pranathi, C. N., Kumar, D. V., & Lakshmi, G. C. (2024, April). Green synthesis of silver nanoparticles using Ocimum tenuiflorum and Azadirachta indica leaf extracts and their antibacterial activity. In Journal of Physics: Conference Series (Vol. 2748, No. 1, p. 012015). IOP Publishing.
- Alzohairy, M. A. (2016). Therapeutic role of Azadirachta indica (Neem) and their active constituents in disease prevention and treatment. Evidence‐Based Complementary and Alternative Medicine, 2016(1), 7382506.
- Vanlalveni, C., Lallianrawna, S., Biswas, A., Selvaraj, M., Changmai, B., & Rokhum, S. L. (2021). Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: A review of recent literature. RSC advances, 11(5), 2804-2837
- Choi, J. S., Jung, H. C., Baek, Y. J., Kim, B. Y., Lee, M. W., Kim, H. D., & Kim, S. W. (2021). Antibacterial activity of green-synthesized silver nanoparticles using Areca catechu extract against antibiotic-resistant bacteria. Nanomaterials, 11(1), 205
- Streeter, K., & Katouli, M. (2016). Pseudomonas aeruginosa: a review of their pathogenesis and prevalence in clinical settings and the environment.
- A Vanlalveni, C., Lallianrawna, S., Biswas, A., Selvaraj, M., Changmai, B., & Rokhum, S. L. (2021). Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: A review of recent literature. RSC advances, 11(5), 2804-2837.
- Rautela, A., & Rani, J. (2019). Green synthesis of silver nanoparticles from Tectona grandis seed extract: characterization and mechanism of antimicrobial action on different microorganisms. Journal of Analytical Science and Technology, 10(1), 1-10.
- Sethuram, L., & Thomas, J. (2023). Synthesis, fabrication, and biosafety profiles of bio-based microemulsion-reinforced electrospun nanofibers for wound dressing applications. Nano-Structures & Nano-Objects, 33, 100940
- Shaik, M. R., Khan, M., Kuniyil, M., Alkhathlan, H. Z., & Adil, S. F. (2018). Plant-extract-assisted green synthesis of silver nanoparticles using Origanum vulgare L. extract and their microbicidal activities. Sustainability, 10(4), 913
- Alzohairy, M. A. (2016). Therapeutic role of Azadirachta indica (Neem) and their active constituents in disease prevention and treatment. Evidence‐Based Complementary and Alternative Medicine, 2016(1), 7382506.
- Zhang, X. F., Liu, Z. G., Shen, W., & Gurunathan, S. (2016). Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. International journal of molecular sciences, 17(9), 1534
- Simon, S., Sibuyi, N. R. S., Fadaka, A. O., Meyer, S., Josephs, J., & Onani, M. O. (2022). Biomedical applications of plant extract-synthesized nanoparticles. Biomedicines, 10(11), 2792.
- Verma, A. D., Jain, N., Singha, S. K., Quraishi, M. A., & Sinha, I. (2016). Green synthesis and catalytic application of curcumin-stabilized Ag nanoparticles. Journal of Chemical Sciences, 128, 1871-1878
- Vorobyova, V., Vasyliev, G., Uschapovskiy, D., Lyudmyla, K., & Skiba, M. (2022). Green synthesis, characterization of silver nanoparticles for biomedical applications and environmental remediation. Journal of Microbiological Methods, 193, 106384.
- Verghese, P. S., and Gurg, M. (2015). Investigation of toxic heavy metals in the drinking water of Agra city, India. Oriental Journal of Chemistry, 31(3), 1835
- Hebeish, A., El-Naggar, M. E., Fouda, M. M., Ramadan, M. A., Al-Deyab, S. S., & El-Rafie, M. H. (2011). Highly effective antibacterial textiles containing green-synthesized Ag silver nanoparticles. Carbohydrate polymers, 86(2), 936-94.
- Daneeh M D, Habil M, and Kumar S Ram. “Innovative approaches in nanomaterials for efficient heavy metal removal from wastewater: A scientific review.”. journal of environmental nanotechnology: Journal of Environmental Nanotechnology 13.4 (2024): 518-526.
- Singh, M., & Verghese, S. (2016). Conventional and innovative techniques for the removal of heavy metals from electroplating industry wastewater. Int J Eng Sci Res Technol, 5(10), 150-159.
- Verghese, S. P. (2016). Studies on sodium diethyl dithiocarbamate for the removal of heavy metals from electroplating industry wastewater and the use of its precipitate as a disease management system for plants. International Journal of Current Research in Chemistry and Pharmaceutical Sciences, 3(2), 1-9.
- Bansal, A., Verghese, S. P., & Singh, R. (2025). Green synthesis of Au nanoparticles using Allium cepa L. (onion) and evaluation of their biological activities. International Journal of Engineering Sciences & Research Technology, 14(1), 34–44
- Singh, R., & Verghese, S. P. (2025). Biosynthesis of selenium nanoparticles (SeNPs) via Curcuma longa extracts and their medicinal applications. In Greening the future: Nanotechnology, environment, and education for sustainable development (pp. 16–34, Chapter 2). Saar Books.
