Introduction:
The International Union of Pure and Applied Chemistry (IUPAC) name of caffeine (chemical formula C₈H₁₀N₄O₂) is trimethylxanthine, commonly referred to as 1,3,7-trimethylxanthine or 3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione. Caffeine is a naturally occurring methylxanthine alkaloid and is widely recognized for its stimulating effects on the central nervous system. It is generally used for the short-term relief of fatigue and for improving alertness and concentration. Once ingested, caffeine is rapidly absorbed in the gastrointestinal tract and quickly distributed into the bloodstream, subsequently crossing the blood–brain barrier to exert its pharmacological effects.
Caffeine is an official drug in both the United States Pharmacopeia (USP) and the British Pharmacopoeia (BP). According to the monographs, methods such as reversed-phase high-performance liquid chromatography (RP-HPLC) have been described for its estimation and quality control. In addition to HPLC, several other analytical techniques have been employed for the determination of caffeine, especially in pharmaceutical formulations and food products. These include UV-visible spectrophotometry, gas chromatography (GC), and mass spectrometry, often in combination with other active pharmaceutical ingredients. However, many of the reported procedures require complex mobile phases containing buffers or involve sophisticated, high-cost instrumentation, which may limit their applicability for routine or resource-limited laboratory settings.
In the present study, efforts are directed towards the development of a simple, cost-effective, and reliable method for caffeine estimation. A solvent extraction followed by gravimetric analysis is proposed, using dichloromethane as the organic solvent for efficient isolation. This approach is advantageous as it avoids the use of complex buffers and minimizes dependence on advanced instruments, making it more accessible for general laboratory use.
Caffeine, in its pure form, is a white crystalline solid with a characteristic melting point of approximately 123 °C. It is moderately soluble in water and can be effectively extracted by boiling aqueous samples. When treated with lead acetate solution, interfering substances such as glucosides are precipitated, leaving caffeine in the filtrate due to its higher solubility in water. This selective precipitation and solubility property form the basis for its isolation and purification from various pharmaceutical and food samples.
Methodology:
A precisely measured quantity of the pharmaceutical and food samples was accurately weighed and transferred into a suitable container. Approximately 100 cm³ of distilled water was added to the samples, which were then heated gently for 15–20 minutes to ensure complete dissolution of all water-soluble constituents. Following this, the resulting hot aqueous solution was filtered to remove any insoluble impurities and particulate matter. To remove any glucoside compounds present in the solution, a saturated solution of lead acetate was added gradually while continuously stirring, until no further precipitate formation was observed. The mixture was then maintained under gentle heating for an additional 5 minutes to ensure the complete precipitation of glucosides. The precipitated glucosides were subsequently separated from the solution by filtration, yielding a clear filtrate.
The obtained filtrate was concentrated carefully under controlled conditions to reduce its volume without causing decomposition of thermally sensitive compounds. The concentrated solution was then subjected to liquid–liquid extraction using dichloromethane (CH₂Cl₂) to isolate caffeine. The extraction process was repeated three times, each time using 15 cm³ of dichloromethane, to maximize the transfer of caffeine from the aqueous phase to the organic phase. After each extraction, the organic layers containing caffeine were combined and transferred into a pre-weighed crucible. The solvent was then evaporated cautiously to dryness under controlled conditions, and the remaining residue was allowed to cool to room temperature before weighing.
The resulting white crystalline residue, representing the caffeine content, was accurately weighed. Finally, the percentage of caffeine present in the original pharmaceutical and food samples was calculated by comparing the weight of the extracted caffeine to the initial weight of the sample, providing a precise quantification of caffeine content.
Figure 1: Weight of different sample in gram
Result and Discussion:
The concentration of caffeine present in various pharmaceutical and food samples was determined, and the results are summarized in Table 1. The percentage of caffeine varies significantly across different products, reflecting the natural and processed sources of caffeine in these items.
Table 1: Percentage of Caffeine in Selected Pharmaceutical and Food Samples
| Sr. No | Sample Description | Caffeine Content (%) |
| 1 | Tobacco-1 | 0.690 |
| 2 | Tobacco-2 | 5.000 |
| 3 | Chocolate-1 | 15.692 |
| 4 | Chocolate-2 | 19.560 |
| 5 | Cold Drink | 5.491 |
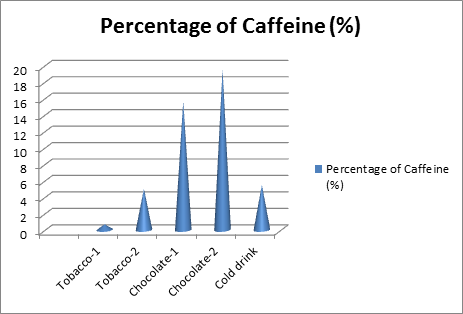
Figure 2: Percentage of Caffeine in different samples
Conclusion:
The maximum recommended daily caffeine intake varies by age. For children aged 4–6 years, the recommended limit is 45 mg per day, while for those aged 10–12 years, it is 85 mg per day (6). For healthy adults, a daily caffeine intake of up to 400 mg is generally considered safe (6).
Analysis of the samples revealed that most of them contained caffeine levels exceeding the standard recommended values, with the exceptions of Tobacco 1 and Tobacco 2, which were within safe limits. In Bhiwandi city, a significant portion of the population regularly consumes various forms of caffeine, including tobacco, coffee, and soft drinks, often at levels that may pose health risks.
To minimize potential health hazards, it is advisable to limit the intake of coffee and soft drinks, ensuring that caffeine consumption remains within the recommended limits set by health authorities. Additionally, the use of tobacco should be completely avoided, as it is associated with numerous harmful effects and the development of serious diseases.
Reference:
1) Chowdhury, S.R.; Maleque, M.; Shihan, M.H. Asian J. Pharm. Ana. 2012, 2, 1-4.
2) Baucells, M.N; Ferrer, P.; Gomez, G.; Lacort, M.; Roura. J. of Molecular Structure. 1993, 294, 219-222.
3) Pascual, M.M.C.; Llobat, E.M.; Roig, M.M.I. Pharmazie, 2000 , 5, 362-3.
4) http://www.bbc.co.uk/news/magazine-22530625
5) Bhawani,S.A.; Fong,S.S.; Mohamad, N.M.I. International J. of Analytical Chem., 2015, 7.
6) https:// www.mayoclinic-org>caffeine
