INTRODUCTION
Oil fuel (BBM) has been one of energy resources that take spotlight of the world’s community due to its increasing consumption every year.
Renewable energy resources such as vegetable oil can be alternative energy solutions as a substitute for fuel oil. By implementing catalytic hydrocracking process, triglyceride in vegetable oil can be converted into hydrocarbon in the form of gasoline and diesel1. The composition of vegetable oil without its content nitrogen, sulphur and heavy metal, will result in environmentally friendly oil fuel.
The main component of castor oil is risinoleic acid (~90%) with little of oleic acid and palmitic acid. The existence of hydroxyl group on risinoleic acid causes castor oil having different characteristic with other vegetable oil and it is fascinating to study. The studies about vegetable oil hydrocracking become hydrocarbon that have been carried out i.e. palm oil1,2, olive oil2, and castor oil2,3. The results of research have shown that vegetable oil can be converted into oil fuel consist of gasoline, diesel, and kerosene.
Impregnating catalyst can be in form of zeolite, alumina, silica, and clay. Clay is fascinating to observe further since its chemical and physical properties can be modified with multiple methods, furthermore the developed catalysts in hydrocracking process of oil are catalysts with big pore size such as zeolite (~8 Å) that is able to cracking fraction of heavy oil. The clay with distributed pillar creates two dimensional form to establish mesoporous material. One of pillaring agents that can be used are oxide metal with high thermal stability that results in increasing thermal stability of bentonite. Oxide metal itself is catalyst, therefore the use of oxide metal has double function i.e. as both pillaring agent and catalyst. Several oxide metal used as pillaring agents are, Al2O35-7, ZrO28-14, TiO215, Fe2O316 and Cr2O317. Among those oxide metals zirconium is special pillaring agent with good thermal stability (700-800 °C) and ability to increase basal spacing to 19-24 Å4.
Study in synthesis on impregnation of Cr metal on ZrO2-pillared bentonite for application of castor oil hydrocracking has not ever been carried out. This study will learn the effect of weight percentage of Cr towards the properties of ZrO2-pillared bentonite and its application to the hydrocracking reaction of castor oil. The combination between Cr as catalyst with ZrO2 both as pillaring agent and catalyst on bentonite as impregnating material is expected to result in the catalyst with its best properties that will be able to convert castor oil into gasoline fraction in maximal result.
MATERIAL AND METHODS
Material that was used including natural bentonite (Beijing Zhongjing Pets Products Co. Ltd), castor oil (Ricinus communis), zirconyl chloride octahydrate (ZrOCl2.8H2O), Chromium nitrate nonahydrate Cr(NO3)3.9H2O, fluoride acid, and silver nitrate. The laboratory equipment includes hydrocracking reactor and cooler, glassware, magnetic stirrer, analite balance, mortar, sieve (250 mesh), and furnace. Analytical instrument consists of Fourier Transform Infrared (FT-IR), X-ray Diffractometer, Atomic Absorption Spectrophotometer, Gas Sorption Analyser (BET), Gas Chromatography-Mass Spectrometry, and Transmission Electron Microscopy.
Preparation of bentonite
Bentonite was immersed in aquadest for 24 hours. The obtained sediment was dried up using oven (100 °C) then immersed in HF solution 1% for 10 minutes, the result was neutralized and dried up with oven (100 °C) then crushed and sifted using sieve at 250 mesh. The sample was then called HF Bentonite.
Preparation of oligo-cation zirconium solution and ZrO2 pillarization
Pillaring solution of oligo-cation zirconium was synthesized using hydrolysis method. Solution of 240 mL ZrOCl2.8H2O 0,1 M was refluxed for 2 hours then added by HF bentonite slowly with ratio of Zr/bentonite at 3 mmol/g and stirred for 24 hours at room temperature. The Zr-intercalated bentonite was neutralized. Sample was dried up in oven (70 °C) then crushed and sieved (250 mesh). Sample was calicinated at temperature of 400 °C for 2 hours. Sample is called as pillared-ZrO2 Bentonite or ZrO2-bentonite.
Impregnation of Cr on ZrO2-Bentonite
Metal impregnation of Cr on ZrO2-bentonite using wet impregnation method. Cr(NO3)3.9H2O was added with ZrO2-bentonite suspension with variation of 1, 2 and 3% (w/w). Sample was refluxed for 5 hours, and then dried up oven (80°C). It took calcinations at temperature of 500 °C for 5 hours, then reducted by H2 gas at temperature of 400 °C for 2 hours respectively with flow rate at 20 mL/minute. The obtained catalyst named as Cr/ZrO2-B-1, Cr/ZrO2-B-2, and Cr/ZrO2-B-3 respectively were Cr/ZrO2-bentonite with Cr-weigh variation of 1, 2 and 3%.
Activity and selectivity examination of Cr/ZrO2-bentonite catalyst
Activity and selectivity examination of catalyst were carried out. Hydrocracking reaction of castor oil with ratio of catalyst towards oil was 1:5% (w/w) using upflow fixed-bed system reactor at temperature of 500 °C followed by H2 gas flow at 20 mL/minute. The resulted liquid product was analyzed with GC-MS.
RESULTS AND DISCUSSION
Synthesis of Cr/ZrO2-bentonite
Analysis of cation-exchange capacity (CEC) on interlayer of bentonite indicates that the bentonite purified with HF will suffer a decrease in CEC following pillarization process i.e. 86.4 into 39.2 meq/100g.
At the pillarization process of bentonite, ZrO2-bentonite was synthesized with intercalation process of pillarization agent zirconyl chloride octahydrate by hydrolysis method and resulted zirconium polication [Zr4(OH)8(H2O)16]8+ that entered into interlayer of Na-bentonite to replace balancing cation such as Na+ and Ca2+. Polication would expand interlayer area of bentonite and after calcination process it would transform into zirconium oxide that becomes stable pillar.
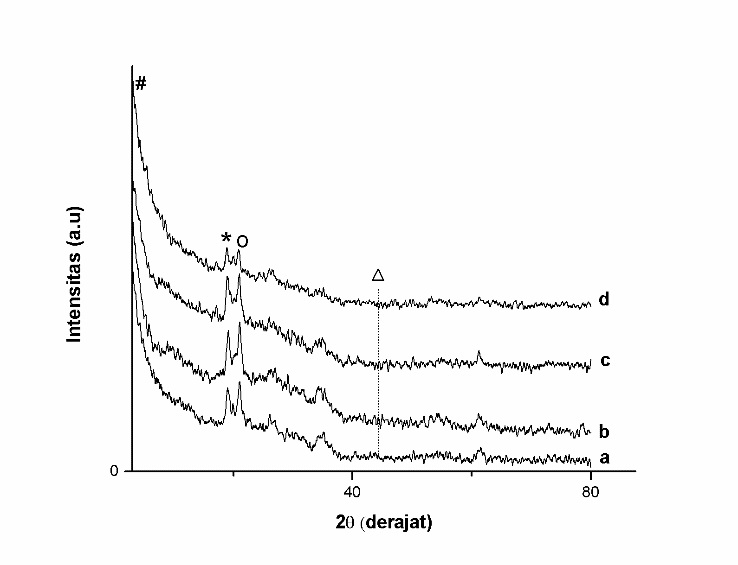
Fig. 1. Diffractogram of ZrO2-bentonite (a), Cr/ZrO2–B-1 (b), Cr/ZrO2–B-2 (c) and Cr/ZrO2–B-3 (d)
Impregnation of Cr on pillared ZrO2-bentonite will decrease basal spacing from peak with d001 characteristic and expand 2θ. The characteristic of peak of Cr metal (JCPDS card No 06-06394) lays on d110 (Fig. 1). The pillarization process can increase basal spacing of bentonite that reflects the success of pillarization process; however the exceeding impregnation process of Cr metal can lower back its basal spacing (Table.2). This can happen because as the impregnation process, the previously established pillars will fall along with the increasing amount of Cr.
FTIR spectra of natural bentonite has specific peaks at wavenumber of 3631 and 3446 cm-1 18. Those peaks appeared at 3626.17 cm-1 in which the absorption band of stretching vibration of Al-OH layer of octahedral structure and 3448.72 cm-1 shows stretching vibration of Si-OH (Figure. 2). After pillarization process and metal impregnation at wavenumber of 3626.17 cm-1 shifting to 3749.62 cm-1 it indicates the bond building between ZrO2 with tetrahedral layer of Si-OH. The wavenumber can also indicate the presence of Si-OH bond that lied at the undetected end of the original bentonite.
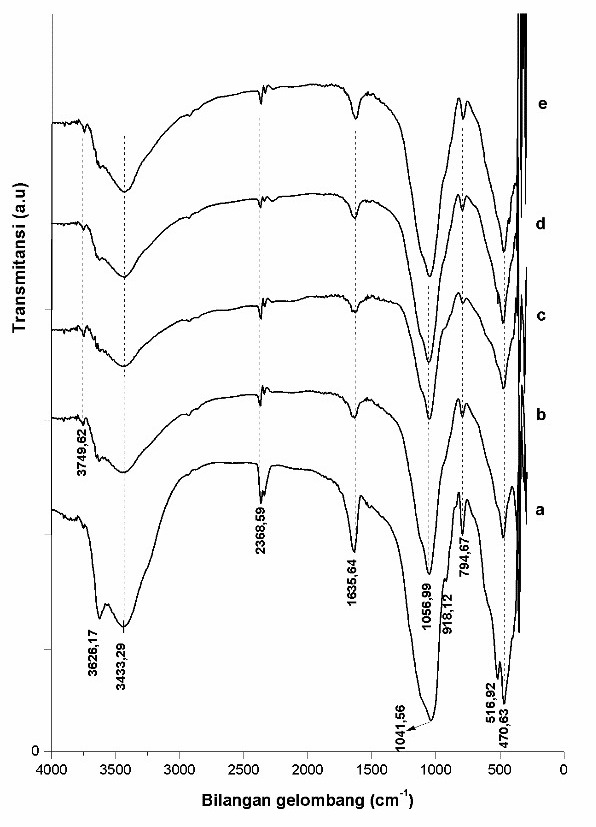
Fig. 2. FTIR spectra of HF Bentonite catalyst (a), ZrO2-bentonite (b), Cr/ZrO2–B-1 (c), Cr/ZrO2–B-2 (d) and, Cr/ZrO2–B-3 (e)
Band absorption of wavenumber at 1635.64 cm-1 reflects bending-vibration OH of water molecule. This increasing intensity indicates that the water-saving capacity and acidity of pillared bentonite have increased due to photon production through dissociation of water molecule. The presence indication of asymmetrical stretching vibration Si-O-Si was shown by the magnitude of band absorption at wavenumber area of 1041.56 cm-1, in which after pillarization and metal impregnation process, it shifted to wavenumber of 1056.99 cm-1. This happened because after the pillarization process there was formation of a bond between O atoms of Si-O-Si with zirconium of the pillar. The number of acidic site in this research was total acidity that included Lewis and Brønsted acid. The presence of oxide metal of ZrO2 and Cr metal on the surface of catalyst will increase the number of active site at the surface of catalyst (Table. 1). Zirconium at the interlayer of bentonite will increase the acidity of solid9. Silanol group (Si-OH) is resulted of the bond disconnection of at tetrahedral layer, through treatment using acid and contact with water contributing towards the Brønsted acidity in clay. The Lewis acidic sites appeared due to the isomorphous substitution on tetrahedral or octahedral layer of clay such as Si4+ by Al3+ and Al3+ by Mg2+ or contribution of Zr and Cr metal on bentonite. The number of acid site is affected by the properties of pillar and condition in synthesis such as reflux’s temperature and mole amount of pillaring agent19, 20.
Table. 1: Results of determination on basal spacing of d001 peak, specific surface area, average porous diameter, total porous volume and acidity of catalyst
| Catalyst | 2θ (˚) | d (Å) | Δd001 (d001-9,6) (Å) | Surface area (m2/g) | Average pore diameter (Å) | Total pore volume (cc/g) | Number of acid site (mmol/g) |
| HF Bentonite | 5.676 | 15.56 | 5.96 | 96.64 | 38.31 | 0.22 | 4.46 |
| ZrO2-bentonite | 3.158 | 27.95 | 18.35 | 164.04 | 38.37 | 0.28 | 7.22 |
| Cr/ZrO2-B-1 | 3.155 | 27.98 | 18.38 | 105.80 | 38.15 | 0.16 | 7.25 |
| Cr/ZrO2-B-2 | 3.535 | 24.97 | 15.37 | 101.07 | 38.49 | 0.17 | 7.78 |
| Cr/ZrO2-B-3 | 3.685 | 23.96 | 14.36 | 102.00 | 38.21 | 0.17 | 8.21 |
*calculated based on ratio % (w/w) of produced liquid fraction
Based on table. 1 it indicates that the specific surface area of bentonite was increasing after pillarization using ZrO2 that indicates the success of pillarization process using ZrO2 on interlayer of bentonite. Impregnation process of active metal on pillared bentonite decreased specific surface area of catalyst that occurred with assumption that while Cr metal entered, it blocked the pores or interlayer and surface on interlayer of bentonite. This was proved by the occurrence of decreasing volume of total pore of catalyst, while the average porous diameter was not going through significant modification.
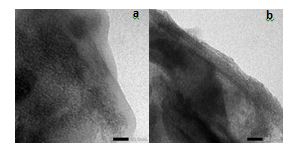
Fig. 3. Morphology of ZrO2-bentonite (a) and Cr/ZrO2–B-3 (b) catalyst
The interlayer arrangement and also the thickness of silicate layer on ZrO2-bentonite and Cr/ZrO2-B-3 was indicated by parallel dark lines reflecting the layered structure of clay (Figure. 3). The homogeneity of interlayer in silicate of Cr/ZrO2-B-3 appeared more identical and the impregnation of Cr metal on ZrO2-bentonite did not ruin the pillars of ZrO2.
Activity and Selectivity Examination of Cr/ZrO2-Bentonite Catalyst
The impregnated Cr metal onto ZrO2-bentonite was analyzed using X-ray fluorescence respectfully resulted in Cr content at 0,26, 0,46 and 0,63% that respectively were Cr-impregnation at 1, 2 and 3%. Activity of catalyst was described by the catalyst performance in converting castor oil into liquid product, gas product and coke. While selectivity of catalyst was described by the catalyst performance to yield gasoline fraction. Product distribution of hydrocracking in castor oil is shown on table. 2.
Table. 2: Conversion result and distribution of liquid product in hydrocracking of castor oil
| Catalyst | Yield (% w/w) | Product (%) | |||
| Liquid | Coke | Gas | Gasoline | Total gasoline | |
| HF Bentonite | 66.23 | 0.32 | 33.46 | – | – |
| ZrO2-bentonite | 65.59 | 0.32 | 34.09 | 22.31 | 28.31 |
| Cr/ZrO2-B-1 | 71.73 | 1.06 | 27.21 | 24.50 | 31.09 |
| Cr/ZrO2-B-2 | 78.80 | 0.94 | 20.25 | 25.79 | 32.73 |
| Cr/ZrO2-B-3 | 77.10 | 0.49 | 22.41 | 25.62 | 32.51 |
Liquid product obtained from Cr/ZrO2-bentonite catalyst was more than the other catalyst. It was caused by the Cr metal that has role as active site therefore it was more optimum to catalyze the hydrocracking reaction. Cr metal actively took role in bond formation and disconnection since it has low energy-half filled orbital. The valence electrons of d orbital were mingled with electrons of s and p orbital that provided great amount of low energy-electronic state in and it is an ideal state to push catalytic reaction. Therefore it is concluded that Cr/ZrO2-bentonite catalyst has the most catalytic activity in converting castor oil in hydrocracking reaction.
Hydrocracking using Cr/ZrO2-B-2 catalyst indicates the highest activity of catalyst in converting castor oil. It can occur due to the overactive catalyst along with the great amount of Cr in cracking castor oil consequently great number of gas phase or light phase will be built and go through perfectly uncondensated saturation, indicated by the higher gas phase.
The liquid product of activity examination of catalyst towards hydrocracking of castor oil with variation in catalyst analyzed using GC-MS. Gasoline fraction is hydrocarbon compounds calculated based on the area of peaks in chromatogram of sample compounds used in hydrocracking of castor oil in accordance with the list of data resulted by GC-MS analysis of pure gasoline of Shimadzu Application Data Sheet No. 21. Selectivity of catalyst towards the gasoline fraction was shown by Cr/ZrO2-B-2 catalyst. The more the gasoline is produced, the better the catalyst is to carry out the catalysis reaction therefore it has good selectivity of catalyst.
Zirconia (ZrO2) pillar after being dehydrated will have Lewis acidic site, in which it contributes as active site in catalysis reactionusing ZrO2-bentonite catalyst. Impregnation of Cr metal into ZrO2-bentonite has huge effect towards the acidic site, in which the more the impregnated Cr metal, the more the number of acidic site on catalyst, thus this situation is very determining in activity and selectivity of hydrocracking of castor oil. Cr metal is group of transition metal that has half-filled electron configuration as the Cr metal performs well if used in multiple catalytic reactions particularly in hydrocracking reaction. d orbital on Cr metal is more effective to weaken σ bond on H2 therefore it has greater catalysis capability compared to the one without metal, this fact is indicated by the greater activity and selectivity of Cr/ZrO2-bentonite catalyst which is ZrO2-bentonite catalyst. The acidity of catalyst affects the product distribution and its selectivity. The greater selectivity towards gasoline fraction using Cr/ZrO2-B-2 bentonite compared to Cr/ZrO2-B-3 catalyst. This likely happen since the impregnation of 3% Cr metal into catalyst enables to increase the number of acid sites, however it is not followed by the increasing strength of its acidic sites or even the strength of its acidic sites are lower compared to Cr/ZrO2-B-2 catalyst, thus the catalyst with greater strength of acidic sites is effective in catalytic hydrocracking process of triglyceride into gasoline fraction. Furthermore Cr/ZrO2-B-2 with the largest pore diameter will ease the larger molecule to enter into pores of catalyst then the hydrocracking process occured and produced smaller molecule such as gasoline fraction.
Hydrocracking reaction of castor oil using catalyst resulted in main product (hydrocarbon) and also uncontrollable products (aldehyde, ketone and carboxylic acid). This situation occurred under physical and chemical interaction involving one or more chemical reaction. Castor oil consists of fatty acid compounds as different ester that has role hydrocracking reaction therefore able to result a number of unwanted products that still contain oxygen. The compound that appeared in hydrocracking product of castor oil that are still the compound mixture among aliphatic, cyclic hydrocarbons, hydrocarbons of aldehyde, ketone, and carbocyclic acid. The highest composition of the product was heptane with its isomer, 1-nonene and 1-heksene.
CONCLUSION
ZrO2-pillared and Cr metal-impregnated bentonite is sufficiently effective in hydrocracking process of castor oil into gasoline fraction. Cr/ZrO2-bentonite catalyst has larger area of natural bentonite. The produced compound of hydrocracking of castor oil resembles gasoline fraction i.e. C6-C11 hydrocarbon.
REFERENCES
- Twaiq, F.A.; Mohamed, A.R.; Bhatia, S., Fuel Process. Technol., 2004, 85, 1283-1300.
- Lappi, H.; Alen, R., J. Anal. Appl. Pyrol.,2011, 91, 154–158.
- Deshpande, D.P.; Haral, S.S.; Sarode, P.B., J. Chem. Sci, 2013, 3(7), 87-89.
- Kloprogge, J.T., J.Por.Mater., 1998, 5, 5-41.
- Fatimah, I. S.; Narsito; Wijaya, K., ITB J. Sci., 2011, 43A, 123-138.
- Garcia, M. L. C.; Galan, L. P.; Ramirez, M. P. S., J. Mex .Chem. Soc., 2006, 50, 36-41.
- Gil, A.; Gandia, L.M.; Vicente, M.A., Catal. Rev-Sci Eng., 2000, 42, 145-212.
- Gil, A.; Vicente, M.A.; Gandia, L.M., Micropor. Mesopor. Mat., 2000, 34, 115-125.
- Torres, E.M.; Farfan; Sham, E.; Grange, P., Catal. Today, 1992, 15, 515-526.
- Fetter, G.; Hernandez, V.; Rodriguez, V.; Valenzuela, M.A.; Lara, V.H.; Bosch, P., Mater. Lett., 2003, 57, 1220–1223.
- Awate, S.V.; Waghmode, S.B.; Agashe, M.S., Catalysis Communications, 2004, 5, 407–411.
- Mishra, B.G.; Rao, G.R., Micropor. Mesopor. Mat., 2004, 70, 43–50.
- Olszewska, D., Appl. Clay Sci., 2011, 53, 353–358.
- Hasanudin; Said, M.; Faizal, M.; Dahlan, M.H.; Wijaya, K.; Sustain. Environ. Res., 2012, 22(6), 395-400.
- Fatimah, I.; Wang, S.; Narsito; Wijaya, K., Appl. Clay Sci., 2010, 50, 588-593.
- Virkutye, J.; Varma, R.S., ACS Sustainable Chem. Eng., 2014, 2, 1545-1550.
- Mata, G.; Trujillano, R.; Vicente, M.A.; Belver, C.; Fernandez-Garcia, M.; Korili, S.A.; Gil, A., Appl. Catal. A Gen., 2007, 327, 1–12.
- Tomul; Fatma., Appl. Surf. Sci., 2011, 258, 1836–1848.
- Kooli, F.; Jones, W., Chem. Mater., 1997, 9, 2913-2920.
- Wijaya, K., Indonesian Journal of Chemistry, 2002, 2, 12-21.
