Introduction: Cucumbers are a popular vegetable crop grown worldwide for their nutritional and culinary value. They are an excellent source of essential vitamins, minerals, and antioxidants. In Nigeria, cucumbers are widely consumed in various forms, including fresh, pickled, and cooked. Ete Market in Nigeria is a major hub for cucumber sales, with various varieties available, including green, white, and African horned cucumbers.
Nutritional values refer to all compounds which are naturally present in foods that exert a specified biological effect on the human body. Recent studies reveal that numerous food wastes and non-edible parts are a good source of nutrients that can be extracted and reintroduced into the food chain as natural food additives [1].
The nutritional values derived from different plants, fruits, and vegetables have been studied to maintain food quality, food safety, and appeal, or as food additives or nutraceuticals to improve nutritional quality and support physiological functions [2].
Fruits and vegetables play a significant role in human nutrition by providing important nutrients including proteins, vitamins, minerals (zinc, calcium, potassium, and phosphorus), fiber, folacin, and riboflavin [3]. The nutritional value varies greatly among fruits and vegetables [4]. It is better to consume a variety of commodities rather than limit consumption to a few with the highest nutritional content. Cucumbers are the most important fruits and vegetables consumed and used as food inform of salad. They are sources of nutrients required for human health [5-7]. The major species of cucumbers growing in Nigeria are Cucumis sativus and Cucumis metuliferus [8]. Cucumis sativus or the cucumber has many varieties, including green and white [9]. Cucumis metuliferus, horned melon, kiwano, and bitter or non-bitter wild cucumber have high economic and nutritional value that is yet to be fully exploited [10]. It has many common names like jelly melon, Kiwano, Melano, and bitter or non-bitter wild cucumber [11]. It is often eaten raw, as a snack, but may also be used in cooking [9].
The nutritional and phytochemical content of different varieties of cucumber fruit is not well known. There is unproven speculations that the nutritional contents of some varieties of cucumber fruits is better than others. This study aimed to assess and compare the nutritional and phytochemical values of three fruits of cucumber varieties with optimal nutritional value sold in Ete market in ikot Abasi local government of Nigeria.
MATERIALS AND METHODS
The fruits Research Design
This study employed a comparative research design to investigate the nutritional content of green, white, and African horned cucumbers sold in Ete Market, Ikot Abasi, Nigeria.
Sampling Strategy
A total of 30 cucumber samples (10 each of green, white, and African horned) were randomly purchased between Januarys – February, 2025 in Ete Market, Ikot Abasi local Government,Nigeria for analysis.
Sample preparation
All the fruits of Green, White and African horned cucumber (Fig. 1) were purchased washed, peeled, and chopped into small pieces using knife before proximate, phytochemical, vitamin, and mineral analyses (Figure 1).


Figure 1: Green (A), white (B) and African horned(C) cucumber fruits and pieces
Nutritional Analysis
The cucumber samples were analyzed for their nutritional content using standard methods [12].
Proximate, Phytochemicals, vitamins, and minerals analysis
The fruit samples were analyzed to determine Proximate (humidity, ash, sugars, proteins, and acidity), phytochemical properties (polyphenols, flavonoids, and tannins), Vitamins (vitamin c), Protein, and Mineral (nitrogen, calcium, magnesium, sodium, potassium, and Iron) contents.
Proximate Analysis
Samples were dried at 1050C in an isothermal oven for three hours and the Ash content was determined. To determine the ash, the samples were incinerated at 525 ± 25 °C for 4 hours using a muffle furnace. Total sugars were evaluated by acid hydrolysis (HCl) by the Luff-Schoorl method [13]. Protein content was determined by the Kjeldahl method [14]. The acidity is the content of organic and mineral acids determined by titration according to the volumetric method.
Phytochemicals Analysis
Analytical methods were used to separate, identify and quantify nutritional components. Polyphenols, flavonoids, and tannins were analyzed by a separation technique using the Spectrophotometric method. 10 ml of the mixture of acetone and water (70/30) was added to 0.5 g of crushed sample and the mixture was agitated for 30mns to homogenize. In each extract of 50μl, 450 μl of distilled water and 2.5 ml of Folin-Ciocalteu were added. 2.5 ml of sodium carbonate is added to each mixture to extract the polyphenol, tannin, and flavonoid compounds. The homogenate mixture was filtered and the filtrates were incubated for 15 minutes at 50°C and subjected to absorbance at 760 nm. The method consists in oxidizing the oxidizable groups of phenols in the basic medium by the method of Folin-Ciocalteu developed by [15]. Tannins were determined by the colorimetric method of Folin Denis, described by [16]. The Flavonoid content of the extracts was determined using the colorimetric method described by [17].
Vitamin C
One gram of the sample was mixed with 10 mL of distilled water and 50mg of oxalic acid. The mixture was titrated with 2.6-DCPIP (Dichlorophenol Indophenol) solution until a persistent pale pink color appears for 30 seconds to determine the vitamin C content [18].
Minerals
Magnesium (Mg), Calcium (Ca), Sodium (Na), Potassium (K), and Iron (Fe) were analyzed
by using Atomic Absorption Spectroscopy (AAS) and Inductively Coupled Plasma Emission (ICP). Nitrogen (N) content was determined by the Kjeldahl method [14].
Statistical analysis
Data collected were subjected to a three-way analysis of variance (ANOVA) performed with SPSS
model 21.0, to determine the main and interaction effects of the studied variables.
Statistical significance was fixed at 0.05. Considering the varieties/parts’ all data are expressed as overall means ± SD.
RESULTS
Proximate and phytochemicals
Proximate and phytochemical screening on cucumber fruit samples was summarized in Table 1 and 2.
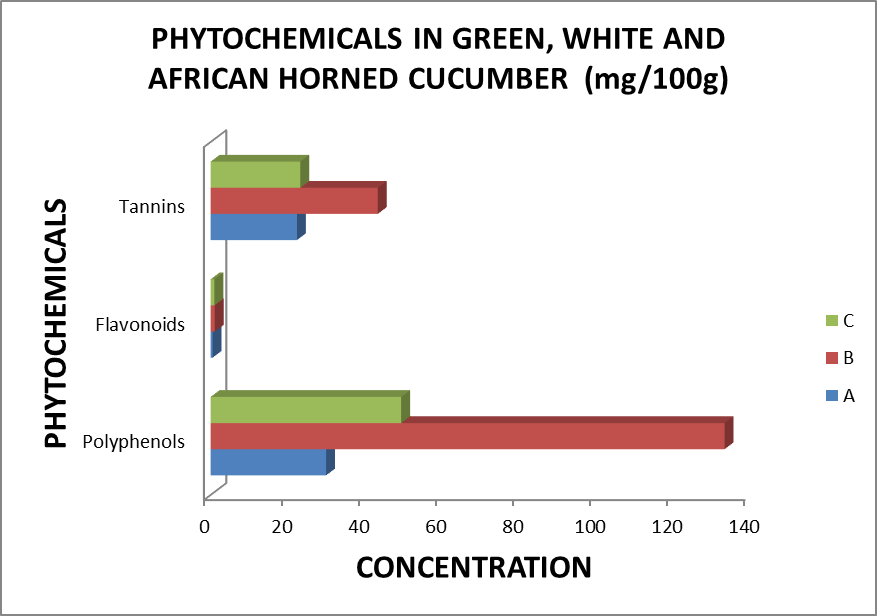
The results showed a relatively high proportion of moisture (varying between 93.76±1.07 and 95.64±0.49%) and sugars (between 430.34±104.91 and 704.57±124.79 mg/100g), a moderate concentration of polyphenols, proteins and tannin (between 22.31±5.93 and 49.28±17.16 mg/100g) and a slightly present of ash and flavonoids (between 0.50±0.11 and 7.52±1.92 mg/100g). There was significant variation in proximate and phytochemical content between varieties. Comparing proximate and phytochemical parameters of the whole fruits of cucumber, the fruit of green C. sativus contained more humidity (96.73±0.49%) than the white variety (95.56±0.70%) and African horned (94.87±1.07%). For ash, white C. sativus (7.52±0.78 mg/100g) and African horned (7.35±1.92 mg/100g) had higher content than green C. sativus (4.62±0.87), while proteins content was significantly high in green C. sativus (35.65±5.12 mg/100g) and white C. sativus (31.86±2.61 mg/100g). The lower content of proteins was recorded in African horned (27.83±2.41 mg/100g). White C. sativus contented significantly more polyphenols (133.05±21.26 mg/100g), flavonoids (1.07±0.46 mg/100g), tannin (43.26±5.18 mg/100g), and sugars (704.57±124.79 mg/100g) than African horned and green C. sativus (Table 1). There was a significant difference in acidity between the fruits of cucumbers with higher acidity content recorded in African Horned cucumber fruits
(6.5±1.45 meq/100g) Table 1.
Table 1: Proximate Composition of the cucumber fruits
| Varieties | Humidity (%) | Ash(mg/100g) | Proteins(mg/100g) | Sugars (mg/100g) | Acidity(meq/100g) |
| A | 96.73±0.49 | 4.62±0.87 | 35.65±5.12 | 440.24±101.90 | 3.01±0.01 |
| B | 95.56±0.70 | 7.52±0.78 | 31.86±2.61 | 704.57±124.79 | 2.80±0.01 |
| C | 94.87±1.07 | 7.35±1.92 | 27.83±2.41 | 469.98±73.42 | 6.50±1.45 |
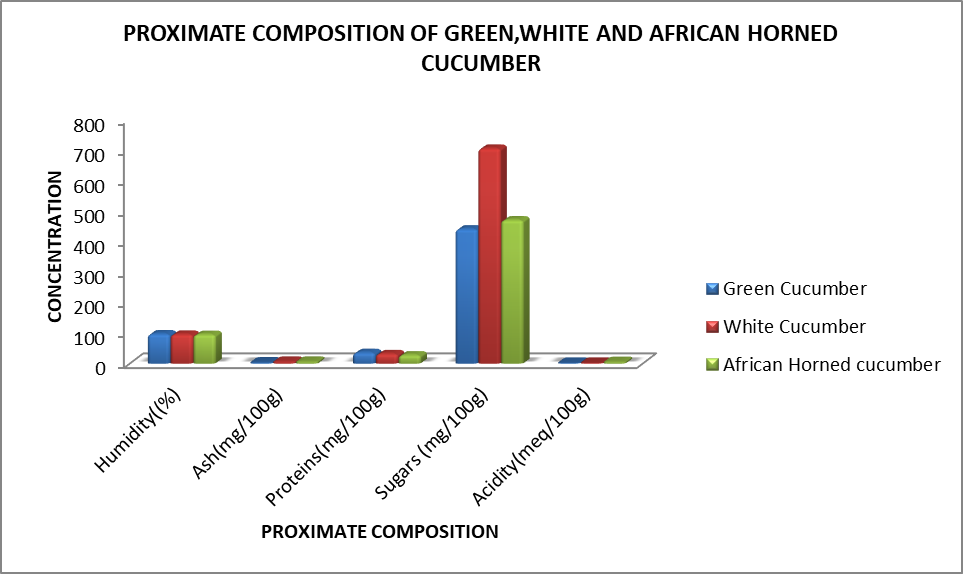
A= Green cucumber, B= white cucumber, C= African horned cucumber
Table 2: phytochemicals
| Varieties | Polyphenols(mg/100g) | Flavonoids(mg/100g) | Tannins(mg/100g) |
| A | 29.89±5.21 | 0.50±0.22 | 22.35±5.92 |
| B | 133.05±21.26 | 1.07±0.46 | 43.26±5.18 |
| C | 49.32±17.15 | 0.96±0.19 | 23.21±3.96 |
Vitamin C
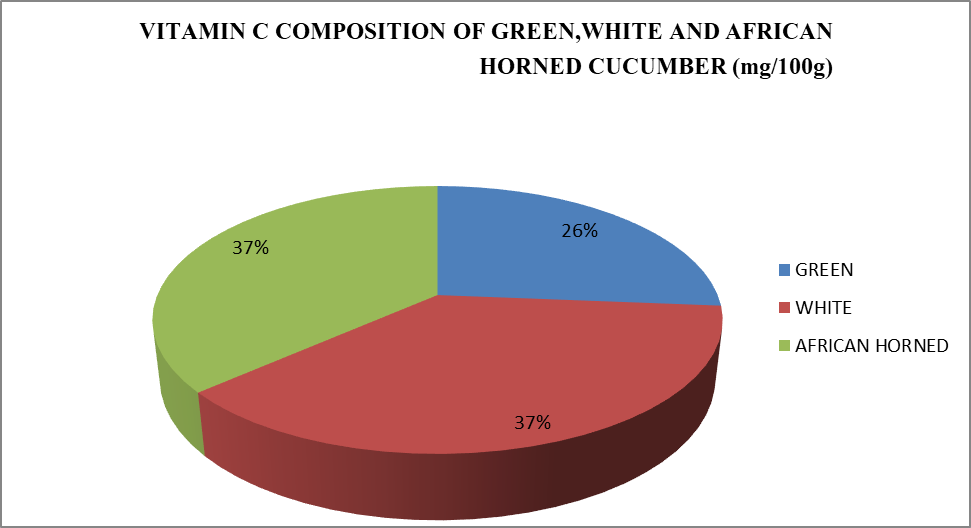
Cucumber fruits are important source of vitamin C varying between 198.05±27.91 and
275.07±44.23 mg/100g (Table 3).There was a little significant difference in vitamin C content between species. However, there was more vitamin C in African horned (275.07±44.23 mg/100g) and white Cucumber (269.57±50.52mg/100g) than the Green cucumber (198.00±27.80).
Table 3: vitamin C content of green, white and African horned cucumber
| Varieties | Vitamin C (mg/100g) |
| A | 198.00±27.80 |
| B | 280.20±44.22 |
| C | 275.07±44.23 |
Minerals
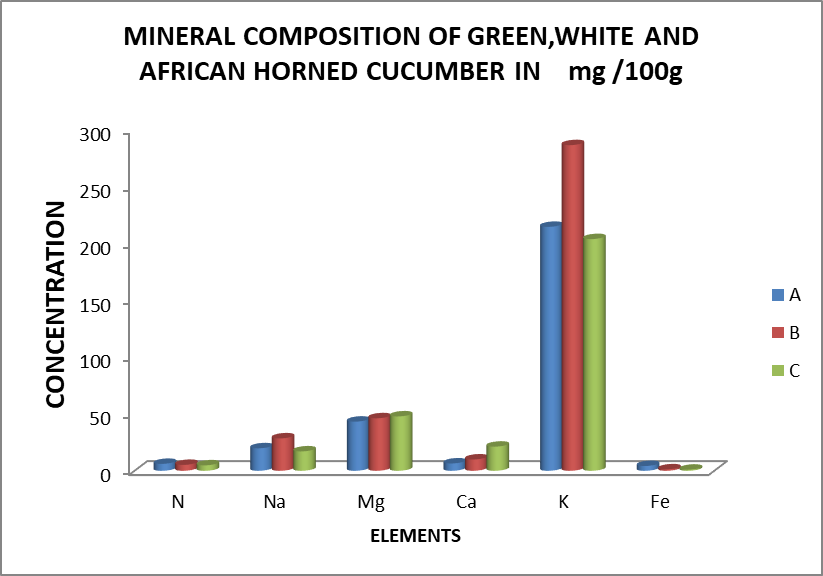
There is no significant difference in Nitrogen (N), Magnesium (Mg), Calcium (Ca), Potassium (K),and iron (Fe) content between varieties was recorded (Table 4). In absolute value, African horned fruit contented more Mg (47.87±10.53 mg/100g) and Ca (21.25±25.40 mg/100g) while higher K (286.58±25.40 mg/100g) and Fe (4224±5.44 mg/100g) were recorded respectively in white and green. There was more Nitrogen in green C. Sativus (5.70±0.81%) followed by white C. sativus (5.11±0.41%) and African horned (4.43±0.94%) (Table 4). Sodium (Na) content recorded in white (28.52±1.37 mg/100g) and green C. Sativus (19.89±5.98 mg/100g) were significantly higher than African horned (17.02±2.51 mg/100g) (Table 4).
| Varieties | N | Na | Mg | Ca | K | Fe |
| A | 6.10±0.03 | 19.80±5.68 | 43.27±10.23 | 6.47±2.58 | 214.70±110.32 | 4.22±5.44 |
| B | 5.20±0.03 | 28.52±1.37 | 46.21±12.18 | 9.97±12.23 | 286.58±25.40 | 1.28±1.06 |
| C | 4.80±0.02 | 17.06±2.52 | 47.87±10.53 | 21.25±25.40 | 203.91±82.90 | 1.00±0.41 |
Discussion
Proximate, physicochemical, vitamin C and minerals content of fruit
Proximate and phytochemical analysis on cucumber fruit samples showed a relatively high proportion of humidity and sugars, a moderate concentration of polyphenols, proteins, and tannins, and a slightly present of ash and flavonoids. For vitamins and minerals, highconcentrations of N and K, and moderate and slight concentrations of Mg, Na, Ca, and Fewere recorded. The high humidity of the whole cucumber (C. sativus and C. metuliferus) fruitvarying between 89 and 96.4% was recorded [6, 19-21]. Cucumber is a rich source of importantnutrients and bioactive compounds; it has been used not only as food but also in therapeuticmedicine and as an ornamental plant [22-24].Cucumber considered a fruit and vegetable crop is rich in polyphenolics and otherphytochemicals [19]. Uthpala [25] have conducted aphytochemical screening on cucumber (C. sativus) homogenate samples and they have foundthat relatively higher amounts of steroids, terpenoids, glycosides, and resins are present incucumber while moderate amounts of saponins, alkaloids, and flavonoids have been reported. Quantitative amounts of the proximate and phytochemicals found reducing sugars in thehighest amount (574.4mg/g) relatively compared to other phytochemicals followed bypolyphenols (8.51 mg/g), flavonoids (2.14 mg/g), and tannins (1.26 mg/g) were the lowestavailable phytochemicals [19]. The analytical composition of C.metuliferus showed proteins, lipids, sugars, and minerals includingmagnesium with high concentration, calcium, potassium, and iron; vitamin C whichconcentration is four times higher in lemon [26-27]. Thephytochemicals present in the fruit of C. metuliferus revealed the presence of usefulsecondary metabolites such as alkaloids, Flavonoids, saponins, tannins, steroids, andterpenoids [28-30].Comparing the proximate and phytochemicals between varieties, green C. sativuscontented more humidity and proteins than white C. sativus and African horned cucumber.White C. sativus contained significantly more polyphenols, flavonoids, tannins, and sugarsfollowed by non-bitter C. metuliferus. For the acidity and ash, the higher values wererecorded in non-bitter C. metuliferus. For vitamin C and minerals (N, Mg, Ca, K and Fe),there was no significant variation between varieties. But more vitamin C, Mg, and Ca wererecorded in non-bitter C. metuliferus followed by white C. sativus while higher K, Fe, and Nawere recorded respectively in white and green C. sativus. The humidity of the whole fruit ofcucumber varied from 89% for C. metuliferus and 96.4% for C. sativus [6,19- 21, 31].
Higher ash (5 mg/g) of C. metuliferus [21] and lower (0.94 mg/g)of C. sativus [19] were recorded. Agatemor et al [19] found highpolyphenols (8.51 mg/g) and sugars (574.36 mg/g) content in C. sativus fruit than in C.metuliferus with respectively 0.89 mg/g (17) and 16.10 mg/g [32].
Comparing the minerals content between species, Ferrara[20] found higher Mg (40mg/100g) and Fe (1.13 mg/100g) content of C. metuliferus than C. sativus with respectively16 mg/100g and 0.70 mg/100 [19]. These authors found higher content ofK (249 mg/100g) and Ca (15 mg/100g) of C. sativus [19] than C.metuliferus with respectively 123 mg/100g and 13 mg/g [20].
Conclusion
Cucumber fruits are a rich source of important nutrients and phytochemical compounds. The nutritional values of cucumber fruits varied according to the species and variety. Based on the species and varieties, white cucumber was richer in sugars, polyphenols, flavonoids, tannins, sodium, and potassium while green cucumber was rich in proteins and iron while African horned cucumber was rich in acidity, vitamin C, magnesium, and calcium. Cucumbers are recognized as fruits and vegetables with multiple nutritional values including, proteins, polyphenols, flavonoids, tannin, sugars, vitamin C and minerals. In Nigeria, cucumber fruit is consumed as a healthy food by few and for others only when recommended by medical personnel. The knowledge of the nutritional value of each fruit species was necessary for better understanding. It is important to consume the fruit to maximize the nutrient supply due to their high nutritional value. In other words, the study highlights the nutritional benefits of consuming different cucumber varieties. The results provide valuable information for consumers, farmers, and nutritionists. Further studies are recommended to investigate the nutritional content of other cucumber varieties and to explore their potential health benefits.
Conflict of interest
The authors declare no conflict of interest to report.
Acknowledgements
We are grateful to National Research Institute for Chemical Technology (NARICT), Zaria, and Kaduna state for the research analysis.
Funding: This research was not funded
References
[1] Vilas-Boas, A. A., Pintado, M., & Oliveira, A. L.(2021). Natural bioactive compounds from foodwaste:Toxicityandsafetyconcerns.Foods,10(7),1564.https://doi.org/10.3390/foods100 71564
[2] Šeregelj, V., Pezo, L., Šovljanski, O., Lević, S., Nedović, V., Markov, S., .and Ćetković, G. (2021). A new concept of fortified yogurt formulation with encapsulated carrot waste extract. LWT, 138,110732. https://doi.org/10.1016/j.lwt.2020.110732
[3] Wargovich, M.J. (2000).Anticancer properties of fruits and vegetables. HortScience, 35, 573-575. https://doi.org/10.21273/HORTSCI.35.4.573
[4] Prior, R. L., and Cao, G. (2000). Antioxidant phytochemicals in fruits and vegetables: diet and healthimplications.HortScience,35(4),588592.https://doi.org/10.21273/HORTSCI.35. 4.588
[5] Sheela, K., Nath, K. G., Vijayalakshmi, D., Yankanchi, G. M., & Patil, R. B. (2004). Proximate composition of underutilized green leafy vegetables in Southern Karnataka Journal of Human Ecology, 15(3), 227-229. https://doi.org/10.1080/09709274.2004.11905698
[6] Mukherjee, P. K., Nema, N. K., Maity, N., and Sarkar, B. K. (2013). Phytochemical andtherapeuticpotentialofcucumber.Fitoterapia,84,227-236. https://doi.org/10.1016/j.fitote.2012.10.003
[7] Deguine JP, Atiama-Nurbel T, Aubertot JN, Augusseau X, Atiama M, Jacquot M, Reynaud B.(2015)Agro-ecological management of cucurbit-infesting fruit fly: a review. Agronomy for Sustainable Development, 35(3), 937–965. https://doi.org/10.1007/s13593-015-0290-5
[8] Diop, A., Sarr, S. O., Sall, A. B., Niass, O., Ndiaye, B., & Diop, Y. M. (2020). Nutritional and antioxidant potential of seeds from two Cucurbitaceae species from Senegal. European Journal of Chemistry, 11(4), 364-369. https://doi.org/10.5155/eurjchem.11.4.364-369.2046
[9] Burkill, H.M. (1985). Useful Plants of West Tropical Africa. Vol.1, 2nd ed. Royal Botanic Gardens,London, 570-605.
[10] Aliero, A. A., and Gumi, A. M. (2012). Studies on the germination, chemical composition, and antimicrobial properties of Cucumis metuliferus. Annals of Biological Research, 3(8), 4059- 4064.
[11] Vieira, E. F., Grosso, C., Rodrigues, F., Moreira, M. M., Fernandes, V. C., & Delerue- Matos, C. (2020). Bioactive Compounds of Horned Melon (Cucumis metuliferus E. Meyer ex Naudin). Bioactive Compounds in Underutilized Vegetables and Legumes, 1-21. https://doi.org/10.1007/978-3-030-44578-2_21-11
[12] AOAC (2005) Official method of Analysis. 18th Edition, Association of Officiating Analytical Chemists, Washington DC, Method 935.14 and 992.24.
[13] Marrubini, G., Papetti, A., Genorini, E., & Ulrici, A. (2017). Determination of the sugar content in commercial plant milk by near-infrared spectroscopy and Luff-Schoorl total glucose titration.Food Analytical Methods, 10(5), 1556-1567.
[14] Sáez-Plaza, P., Michałowski, T., Navas, M. J., Asuero, A. G., & Wybraniec, S. (2013). An overview of the Kjeldahl method of nitrogen determination. Part I. Early history, the chemistry of the procedure, and titrimetric finish. Critical Reviews in Analytical Chemistry, 43(4), 178-223.https://doi.org/10.1080/10408347.2012.751786
[15] Georgé, S., Brat, P., Alter, P., & Amiot, M. J. (2005). Rapid determination of polyphenols and vitamin C in plant-derived products. Journal of Agricultural and Food Chemistry, 53(5), 1370-1373. https://doi.org/10.1021/jf048396b
[16] Joslyn, M. A. (1970) Ash Content and Ashing Procedures. Methods in Food Analysis. Physical,Chemical, and Instrumental Methods of Analysis. Second Edition, Academic Press, New York:109-140.
[17] Kim, D. O., Chun, O. K., Kim, Y. J., Moon, H. Y., & Lee, C. Y. (2003). Quantification of
polyphenolics and their antioxidant capacity in fresh plums. Journal of Agricultural and Food Chemistry, 51(22), 6509-6515. https://doi.org/10.1021/jf0343074
[18] Nielsen, S. S., and Nielsen, S. S. (2017). Vitamin C determination by indophenol method. Food analysis Laboratory Manual, 143-146. https://doi.or/10.1007/978-3-319-44127-6_15
[19] Agatemor, U. M. M., Nwodo, O. F. C., & Anosike, C. A. (2018). Phytochemical and proximate composition of cucumber (Cucumis sativus) fruit from Nsukka, Nigeria. African Journal of Biotechnology, 17(38), 1215-1219. https://doi.org/10.5897/AJB2018.16410
[20] Ferrara L.(2006) The dietary importance of tropical fruit: the kiwano. Ingredienti Alimentari, 5,14–17.
[21] Romero-Rodriguez, M. A., Vazquez-Oderiz, M. L., Lopez-Hernandez, J., and Simal-Lozano, J. (1992). Physical and analytical characteristics of the kiwano. Journal of Food Composition and Analysis, 5(4), 319-322. https://doi.org/1016/0889-1575(92)90065-r
[22] Dixit, Y. and Kar, A. (2010). Protective role of three vegetable peels in alloxan-induced diabetes mellitus in male mice.Plant Foods for Human Nutrition, 65, 284-289. https://doi.org/10.1007/s11130- 010-0175-3
[23] Kapoor, L. D., (2001). Handbook of ayurvedic medicinal plants. CRC Press.
[24] Uthpala, T. G. G., Marapana, R. A. U. J., Lakmini, K., and Wettimuny, D. C. (2020). Nutritional bioactive compounds and health benefits of fresh and processed cucumber (Cucumis sativusL.).Sumerianz Journal of Biotechnology, 3(9), 75-82.
[25] Uthpala, T. G. G., Marapana, R. A. U. J., and Jayawardana, S. A. S., (2018). Sensor quality and physicochemical evaluation of two brine pickled cucumber (Cucumis sativus L.) varieties.International Journal of Advanced Engineering Research and Science, 5, 22- 26.
[26] Usman, J. G., Sodipo, O. A., Kwaghe, A. V., & Sandabe, U. K. (2015). Uses of Cucumis metuliferus: a review. Cancer Biology, 5(1), 24-34.
[27] Hussein, A. H. A. (2009). Impact of sewage sludge as organic manure on some soil properties, growth,yield, and nutrient contents of cucumber crop. Journal of Applied Sciences, 9(8), 1401- 1411. https://doi.org/10.3923/jas.2009.1401.1411.
[28] Jimam, N. S., Wannang, N. N., Anuka, J. A., Omale, S., Falang, K. D., & Adolong, A. A. (2011). Histopathologic effect of C. Metuliferus E Mey (CUCURBITACEAE) fruits in albino rats.International Journal of Pharmaceutical Sciences and Research, 2(8), 2190-2194. http://doi.org/10.13040/IJPSR.0975-8232.2(8).2190-94
[29] Gotep, J. (2011). Glycosides fraction extracted from the fruit pulp of Cucumis metuliferus E. Meyer has an antihyperglycemic effect in rats with alloxan-induced diabetes. Journal of Natural Pharmaceuticals, 2, 48-51
[30] Usman, J. G., Sodipo, O. A., & Sandabe, U. K. (2014). Phytochemical screening and acute toxicity study of Cucumis metuliferus E. Mey. Ex. Naudin fruit extract in cockerels. International Journal of Phytomedicine, 6(2), 243-247.
[31] USDA (2015) The plants’ database. United States Department of Agriculture – Natural Resources Conservations Service. http://plants.usda.gov. Accessed 28 January 2023
[32] Benzioni, A., Mendlinger, A., Ventura, M., and Huyskens, S. (1993). Germination, fruit development, yield, and post-harvest characteristic of Cucumis metuliferus. New York: New Crops Wiley, 553-557.
