Introduction: Molecules are a collection of two or more atoms held together by a chemical bond. Molecules may be homonuclear, contain atoms of one chemical element, such as two atoms in the oxygen molecule, or they may be heteronuclear, consisting of more than one chemical element, such as a molecule of water since it has two different atoms. A covalent bond is a chemical bond that contains the sharing of electron pairs between atoms.
A chemical bond is a collection of atoms or ions that constitute molecules and crystals. The chemical bond may be attributed to the electric force between charged ions with opposite signs, as in ionic bonds, or by the sharing of electrons, as in covalent bonds. The polarity, directionality and the strength of bonds can be determined through octet rule, theory of Valence Shell Electron Pair Repulsion (VSEPR), and the theory of Valence Bond (VB), which contains orbital hybridization, resonance and molecular orbital theory, which comprises a linear combination of ligand field theory and atomic orbitals [1- 6].
Covalent radius is the smallest distance between two atoms bonded together with sharing of electrons. It is the distance between two atoms approaching each other without interfering. The sum of the two covalent radii is equal to the covalent bond length between two atoms. The bond lengths are measured by neutron diffraction on molecular crystals or X-ray diffraction. Rotational spectroscopy gives precise values of bond lengths [7 – 10].
Small systems such as atoms and molecules have constant values because of the homogenous distribution of their constituents. It is found that the atom as a nucleus and electrons at certain distances are contained in a certain area with a certain uniform distribution forming a certain system (atom) with a certain constant (1.1 X 108 m2/Kg) [11]. The same result with certain constant (5.4 X 109 m-1) is found for homonuclear molecules as a sharing of electron pairs between two similar atoms with uniform distribution to form certain covalent chemical bonds.
Materials and Methods
Physical relations and laws can be expressed regarding the main physical parameters responsible for their origin. The shared electrons between two atoms forms a covalent bond with a certain uniform distribution, producing a certain uniform system (molecule).
The main goal of collective covalent radii is to estimate approximately as given in equation (1), the length of a bond as the sum of two atomic radii

The origin for these covalent radii for single bonds is found in [12]. The list of metallic bonding and VCH periodic table is reported in [13,14]. In simple terms, a single bond corresponds to an electron pair in a bonding molecular orbital.
The covalent radii in pure-bred shape are determined by bisecting a homonuclear bond, as shown in the following equation (2).
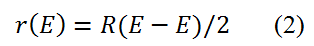
The new group of single bond covalent radii for the elements 1 – 96, based on enormous data mining from the Cambridge Structural Data Base (CSD) and estimating the carbon radii (separately for the three familiar hybridizations), nitrogen and oxygen is found in [15].
The present scheme and method to determine the covalent radii are different. We considered diatomic homonuclear molecules as equal in their spherical geometrical shape. All covalent radii are then obtained self-consistently by using a simple equation connecting the radius, which represents the covalent radius and several sharing electrons. Our approach confirms that the diatomic homonuclear molecules have spherical shape and its mathematical relation can be used to calculate covalent radii and confirm certain constant (5.4 X 109 m-1)
Results and Discussions
Density Functional Theory (DFT) is a powerful method utilized in both physics and chemistry to investigate the electronic structure (especially the ground state) for atoms and molecules. Using this theory, the characteristics of a many-electron arrangement can be estimated. In spite of late improvements, there are some difficulties in using DFT to describe intermolecular interactions such as realizing chemical reactions, particularly van der Waals forces [16-17].
Molecular Orbital (MO) theory provides a method to describe the electronic structure of molecules by using molecular quantum mechanics or quantum chemistry. MO theory treating the states of bonded electrons – the molecular orbitals – as Linear Combination of Atomic Orbitals (LCAO) by using the models of Density Functional Theory (DFT) to the Schrodinger equation. These are represented by three kinds (bonding, antibonding, non – bonding). A bonding orbital intensifies electron density in the area between a particular couple of atoms, consequently its electron density will tend to pull each of the two nuclei toward the other and hold the two atoms together [18 – 19].
The participation of electrons between two atoms to form covalent bonds leads to the formation of particular molecules. Diatomic homonuclear molecules are formed with certain geometrical shape (spherical) with a certain circumference for each molecule. It is found that the covalent radii of this type of molecules behave linearly as a result of increasing atomic number in periodic table. The model is based on the number of sharing electrons and the circumference and both of them are increasing with increasing atomic number.
It is found that the proportionality between cubic root of the number of shared electrons and circumference for diatomic homonuclear molecules gives the accurate results with experimental values equations as shown in equations (3 & 4 & 5)
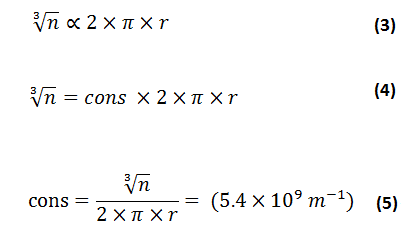
From which the following equation can be deduced
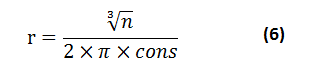
The calculated covalent radius of homonuclear molecules by using equation (6) is consistent and in good agreement with experimentally determined radii values [20] as indicated in Table 1
where
n is the number of electrons of two atoms
r is the covalent radius
cons. is constant value for molecules
All calculations confirmed that there is a constant value (5.4 X 109 m-1) for molecules formed by covalent bonds relating to the cubic root of the number of electrons and their circumference. Table (1) lists the constant value of similar molecules consisting of one chemical element by using equation (5).
A comparison of the present calculations and results for the covalent radius of diatomic homonuclear molecules with the available experimental values shows a good agreement between them [8].
The physical meaning of the constant of diatomic homonuclear molecules represents the number of sharing electrons to form certain covalent bond and radius of spherical geometrical shape of this type of molecules.
There are only seven calculated covalent radii approximately 5 % from 118 molecules by using equation (3) are deviated from the experimental determined values for the elements (Lithium, Beryllium, Sodium, Magnesium, Aluminium, Silicon, Phosphorus). The difference may be referred to the deviation of these molecules from spherical geometrical shape.
Table 1. List the experimental and new covalent radii.
| Atomic Number | Symbol | Name | Experimental Covalent Radius picometer (pm) | New Covalent Radius picometer (pm) | Constant |
| 1 | H | Hydrogen | 32 | 37 | 5.4 X 109 |
| 2 | He | Helium | 46 | 47 | 5.4 X 109 |
| 3 | Li | Lithium | 133 | 54 | 5.4 X 109 |
| 4 | Be | Beryllium | 102 | 59 | 5.4 X 109 |
| 5 | B | Boron | 85 | 63 | 5.4 X 109 |
| 6 | C | Carbon | 75 | 67 | 5.4 X 109 |
| 7 | N | Nitrogen | 71 | 71 | 5.4 X 109 |
| 8 | O | Oxygen | 63 | 74 | 5.4 X 109 |
| 9 | F | Fluorine | 64 | 77 | 5.4 X 109 |
| 10 | Ne | Neon | 67 | 80 | 5.4 X 109 |
| 11 | Na | Sodium | 155 | 82 | 5.4 X 109 |
| 12 | Mg | Magnesium | 139 | 85 | 5.4 X 109 |
| 13 | Al | Aluminium | 126 | 87 | 5.4 X 109 |
| 14 | Si | Silicon | 116 | 90 | 5.4 X 109 |
| 15 | P | Phosphorus | 111 | 92 | 5.4 X 109 |
| 16 | S | Sulfur | 103 | 94 | 5.4 X 109 |
| 17 | Cl | Chlorine | 99 | 96 | 5.4 X 109 |
| 18 | Ar | Argon | 96 | 98 | 5.4 X 109 |
| 19 | K | Potassium | 196 | 99 | 5.4 X 109 |
| 20 | Ca | Calcium | 171 | 101 | 5.4 X 109 |
| 21 | Sc | Scandium | 148 | 103 | 5.4 X 109 |
| 22 | Ti | Titanium | 136 | 104 | 5.4 X 109 |
| 23 | V | Vanadium | 134 | 105 | 5.4 X 109 |
| 24 | Cr | Chromium | 122 | 106 | 5.4 X 109 |
| 25 | Mn | Manganese | 119 | 108 | 5.4 X 109 |
| 26 | Fe | Iron | 116 | 110 | 5.4 X 109 |
| 27 | Co | Cobalt | 111 | 111 | 5.4 X 109 |
| 28 | Ni | Nickel | 110 | 112 | 5.4 X 109 |
| 29 | Cu | Copper | 112 | 114 | 5.4 X 109 |
| 30 | Zn | Zinc | 118 | 115 | 5.4 X 109 |
| 31 | Ga | Gallium | 124 | 116 | 5.4 X 109 |
| 32 | Ge | Germanium | 121 | 117 | 5.4 X 109 |
| 33 | As | Arsenic | 121 | 118 | 5.4 X 109 |
| 34 | Se | Selenium | 116 | 119 | 5.4 X 109 |
| 35 | Br | Bromine | 114 | 121 | 5.4 X 109 |
| 36 | Kr | Krypton | 117 | 122 | 5.4 X 109 |
| 37 | Rb | Rubidium | 210 | 123 | 5.4 X 109 |
| 38 | Sr | Strontium | 185 | 125 | 5.4 X 109 |
| 39 | Y | Yttrium | 163 | 126 | 5.4 X 109 |
| 40 | Zr | Zirconium | 154 | 127 | 5.4 X 109 |
| 41 | Nb | Niobium | 147 | 128 | 5.4 X 109 |
| 42 | Mo | Molybdenum | 138 | 129 | 5.4 X 109 |
| 43 | Tc | Technetium | 128 | 130 | 5.4 X 109 |
| 44 | Ru | Ruthenium | 125 | 131 | 5.4 X 109 |
| 45 | Rh | Rhodium | 125 | 132 | 5.4 X 109 |
| 46 | Pd | Palladium | 120 | 132 | 5.4 X 109 |
| 47 | Ag | Silver | 128 | 133 | 5.4 X 109 |
| 48 | Cd | Cadmium | 136 | 134 | 5.4 X 109 |
| 49 | In | Indium | 142 | 135 | 5.4 X 109 |
| 50 | Sn | Tin | 140 | 136 | 5.4 X 109 |
| 51 | Sb | Antimony | 140 | 137 | 5.4 X 109 |
| 52 | Te | Tellurium | 136 | 138 | 5.4 X 109 |
| 53 | I | Iodine | 133 | 139 | 5.4 X 109 |
| 54 | Xe | Xenon | 131 | 140 | 5.4 X 109 |
| 55 | Cs | Cesium | 232 | 141 | 5.4 X 109 |
| 56 | Ba | Barium | 196 | 142 | 5.4 X 109 |
| 57 | La | Lanthanum | 180 | 143 | 5.4 X 109 |
| 58 | Ce | Cerium | 163 | 144 | 5.4 X 109 |
| 59 | Pr | Praseodymium | 176 | 144 | 5.4 X 109 |
| 60 | Nd | Neodymium | 174 | 145 | 5.4 X 109 |
| 61 | Pm | Promethium | 173 | 145 | 5.4 X 109 |
| 62 | Sm | Samarium | 172 | 146 | 5.4 X 109 |
| 63 | Eu | Europium | 168 | 147 | 5.4 X 109 |
| 64 | Gd | Gadolinium | 169 | 148 | 5.4 X 109 |
| 65 | Tb | Terbium | 168 | 149 | 5.4 X 109 |
| 66 | Dy | Dysprosium | 167 | 149 | 5.4 X 109 |
| 67 | Ho | Holmium | 166 | 150 | 5.4 X 109 |
| 68 | Er | Erbium | 165 | 150 | 5.4 X 109 |
| 69 | Tm | Thulium | 164 | 151 | 5.4 X 109 |
| 70 | Yb | Ytterbium | 170 | 152 | 5.4 X 109 |
| 71 | Lu | Lutetium | 162 | 153 | 5.4 X 109 |
| 72 | Hf | Hafnium | 152 | 153 | 5.4 X 109 |
| 73 | Ta | Tantalum | 146 | 154 | 5.4 X 109 |
| 74 | W | Tungsten | 137 | 155 | 5.4 X 109 |
| 75 | Re | Rhenium | 131 | 156 | 5.4 X 109 |
| 76 | Os | Osmium | 129 | 156 | 5.4 X 109 |
| 77 | Ir | Iridium | 122 | 157 | 5.4 X 109 |
| 78 | Pt | Platinum | 123 | 157 | 5.4 X 109 |
| 79 | Au | Gold | 124 | 158 | 5.4 X 109 |
| 80 | Hg | Mercury | 133 | 159 | 5.4 X 109 |
| 81 | Tl | Thallium | 144 | 159 | 5.4 X 109 |
| 82 | Pb | Lead | 144 | 160 | 5.4 X 109 |
| 83 | Bi | Bismuth | 151 | 161 | 5.4 X 109 |
| 84 | Po | Polonium | 145 | 161 | 5.4 X 109 |
| 85 | At | Astatine | 147 | 162 | 5.4 X 109 |
| 86 | Rn | Radon | 142 | 162 | 5.4 X 109 |
| 87 | Fr | Francium | 163 | 5.4 X 109 | |
| 88 | Ra | Radium | 201 | 164 | 5.4 X 109 |
| 89 | Ac | Actinium | 186 | 165 | 5.4 X 109 |
| 90 | Th | Thorium | 175 | 165 | 5.4 X 109 |
| 91 | Pa | Protactinium | 169 | 166 | 5.4 X 109 |
| 92 | U | Uranium | 170 | 167 | 5.4 X 109 |
| 93 | Np | Neptunium | 171 | 168 | 5.4 X 109 |
| 94 | Pu | Plutonium | 172 | 169 | 5.4 X 109 |
| 95 | Am | Americium | 166 | 170 | 5.4 X 109 |
| 96 | Cm | Curium | 166 | 170 | 5.4 X 109 |
| 97 | Bk | Berkelium | 168 | 170 | 5.4 X 109 |
| 98 | Cf | Californium | 168 | 171 | 5.4 X 109 |
| 99 | Es | Einsteinium | 165 | 171 | 5.4 X 109 |
| 100 | Fm | Fermium | 167 | 171 | 5.4 X 109 |
| 101 | Md | Mendelevium | 173 | 172 | 5.4 X 109 |
| 102 | No | Nobelium | 176 | 172 | 5.4 X 109 |
| 103 | Lr | Lawrencium | 161 | 173 | 5.4 X 109 |
| 104 | Rf | Rutherfordium | 157 | 173 | 5.4 X 109 |
| 105 | Db | Dubnium | 149 | 175 | 5.4 X 109 |
| 106 | Sg | Seaborgium | 143 | 175 | 5.4 X 109 |
| 107 | Bh | Bohrium | 141 | 176 | 5.4 X 109 |
| 108 | Hs | Hassium | 134 | 176 | 5.4 X 109 |
| 109 | Mt | Meitnerium | 129 | 176 | 5.4 X 109 |
| 110 | Ds | Darmstadtium | 128 | 176 | 5.4 X 109 |
| 111 | Rg | Roentgenium | 121 | 177 | 5.4 X 109 |
| 112 | Cn | Copernicium | 122 | 178 | 5.4 X 109 |
| 113 | Nh | Nihonium | 136 | 179 | 5.4 X 109 |
| 114 | Fi | Flerovium | 143 | 179 | 5.4 X 109 |
| 115 | Mc | Moscovium | 162 | 180 | 5.4 X 109 |
| 116 | Lv | Livermorium | 175 | 180 | 5.4 X 109 |
| 117 | Ts | Tennessine | 165 | 181 | 5.4 X 109 |
| 118 | Og | Oganesson | 157 | 181 | 5.4 X 109 |
It is noticed that all homonuclear molecules have the same value (5.4 X 109 m-1) This means and indicates that all molecules have the same constant value relating to the cubic root of the number of electrons and their circumference.
Conclusion
The homogenous distribution of participating or sharing electrons for diatomic homonuclear molecules formed by covalent bonds with a certain number and certain circumference leads to the existence of a common constant for all diatomic homonuclear molecules. The cubic root of the number of sharing electrons and their circumference gives constant value with calculations and results of covalent radii in good agreement with experimental determinations.
Acknowledgement
“The author would like to express his sincere appreciation and deepest gratitude to the anonymous referee and all the staff of the United Journal of Chemistry whose contribution and support have greatly enhanced the quality, accuracy and publication of this research”
Conflict of interest
The author declares no conflicts of interest.
References
- Levine, D. S.; Martin, H. G. Clarifying the quantum mechanical origin of the covalent chemical bond. Nature Communications. 2020, 11, 4893
- Allen, F. H.; Kennard, O.; Watson, D. G.; Brammer, L.; Orpen, A.G.; Tylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc., Perkin Trans. 1987, 2 (12), S1-S19.
- Frenking, G.; Krapp, A. Unicorns in the world of chemical bonding models. Journal of Computational Chemistry. 2007, 28 (1), 15–24.
- Gillespie, R. Teaching Molecular Geometry with the VSEPR Model. Journal of Chemical Education. 2004, 81 (3), 298-304
- Harwood, W.S.; Herring. F.G, General Chemistry: Principles and Modern Applications (8th ed.). 2002, Prentice-Hall. ISBN 978-0-13-014329-7.
- Bader, R. F. W.; Gillespie, R. J.; MacDougall, P. J. A physical basis for the VSEPR model of molecular geometry. J. Am. Chem. Soc. 1988, 110 (22): 7329–7336.
- Hund, F. Zur Deutung der Molekelspektren. IV. Zeitschrift für Physik. 1928, 51 (11–12), 759 – 795.
- Orpen, A. G.; Brammer, L.; Frank, H. A.; Olga, K.; Watson, D. G.; Tylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 2. Organometallic compounds and co-ordination complexes of the d- and f-block metals. Journal of the Chemical Society, Dalton Transactions. 1989, S1-S83.
- Sanderson, R. T. Electronegativity and bond energy. Journal of the American Chemical Society. 1983, 105 (8), 2259-2261.
- Pauling, L. The Nature of the Chemical Bond. II. The One-Electron Bond and the Three Electron Bond. Journal of the American Chemical Society. 1931, 53 (4), 3225-3237.
- Sanad, M. R. Constant of Atoms and New Equation of Electromagnetic Force. Proceedings of the Romanian Academy, series A. 2024, 25 (4), 301-306.
- Pauling, L. The Nature of the Chemical Bond, 3rd ed., Cornell University Press, Ithaca, NY, 1960.
- Pauling, L; Kamb, B. Proc. Natl. Acad. Sci. USA, 1986, 83, 3569 – 3571.
- Fluck, E; Heumann, K.G. Periodensystem der Elemente, 3rd ed., Wiley-VCH, Weinheim, 2002.
- Cordero, B.; Gómez, V.; Platero-Prats, A, E.; Revés, M.; Echeverría, J.; Cremades, E.; Barragán, F.; Alvarez, S. Covalent radii revisited. Dalton Trans. 2008, 2832-2838.
- Assadi, M. H. N.; et al. “Theoretical study on copper’s energetics and magnetism in TiO2 polymorphs”. Journal of Applied Physics. 2013, 113 (23): 233913–233913–5
- Medvedev, Michael G.; Bushmarinov, Ivan S.; Sun, Jianwei; Perdew, John P.; Lyssenko, Konstantin, “Density functional theory is straying from the path toward the exact functional”. Science. 2017, 355 (6320): 49–52
- Miessler and Tarr, Inorganic Chemistry, 2013, 5th ed, 117-165, 475-534
- Albright, T. A.; Burdett, J. K.; Whangbo, M.-H. Orbital Interactions in Chemistry. 2013, Hoboken, N.J.: Wiley.
- Pyykkö, P.; Atsumi, M. Molecular Single-Bond Covalent Radii for Elements 1–118. Chemistry: A European Journal. 2009, 15 (1), 186-197.
