INTRODUCTION
Water pollution continues to be a critical global issue, impacting ecosystems, human health, and overall environmental sustainability. Among the various pollutants, nitrate contamination in water bodies poses serious challenges for water quality management. Nitrate pollution, often stemming from agricultural runoff and wastewater discharge, has been linked to adverse health effects, including methemoglobinemia or “blue baby syndrome” in infants, as well as eutrophication in aquatic environments.
To mitigate health risks, the World Health Organization (WHO) has set a guideline limit of 50 mg/L of nitrate in drinking water, while the Bureau of Indian Standards (BIS) recommends a slightly lower limit of 45 mg/L. Nitrate ions are highly soluble in water and exhibit resonance stabilization, which makes their removal particularly challenging.
Several conventional methods have been explored for nitrate removal, such as reverse osmosis, ion exchange, electrodialysis, biological denitrification, and chemical reduction. However, these approaches often face limitations in terms of efficiency, sustainability, or operational cost, prompting the need for alternative, eco-friendly techniques. Among them, adsorption is widely recognized as a promising method due to its simplicity, operational convenience, and cost-effectiveness.
The effectiveness of adsorption largely depends on the properties of the adsorbent used. Various materials, including activated carbon, alumina, silica, charcoal, nanoparticles, ion exchange resins, fly ash, zeolites, and agricultural wastes, have been investigated for nitrate adsorption. Commercial anion exchange resins such as Imac HP555, Indion NSSR, Purolite A100, Amberlite IRN-78, and Amberlite IRA 400 have demonstrated high nitrate removal capacities, albeit with high associated costs.
To address this, low-cost adsorbents derived from agricultural or biomass waste are being developed, owing to their beneficial physicochemical properties and abundant availability. Despite their potential, raw agricultural materials often exhibit limited adsorption capacities due to their inherent chemical structure. Therefore, surface modification—either physical or chemical—is often employed to enhance their adsorption performance.
Techniques such as protonation, metal ion impregnation, and amine grafting have been used to introduce positive surface charges, thereby improving the adsorbent’s affinity for anionic pollutants like nitrate. For example, iron-impregnated Dowex 21 XLT demonstrated improved nitrate adsorption due to increased surface charge. Similarly, amine-functionalized corn cob and coconut copra showed enhanced performance compared to their unmodified counterparts. Modified reed straw biochar treated with FeCl₃ and HCl-activated sepiolite also exhibited improved nitrate adsorption through increased surface functionality.
In the present study, for the first time, acid-activated Salix alba leaves powder—a locally available biomass—was explored as a novel adsorbent for nitrate removal.
Ladakh, a cold arid region with limited vegetation, hosts several naturally growing Salix species. Salix alba, commonly known as white willow, is a deciduous tree that thrives in this region. Traditionally, its leaves are used as fodder for livestock. Previous studies have employed Salix alba leaves in powdered or biochar form for the removal of dyes such as malachite green, brilliant green, and methylene blue.
The objectives of this study are as follows:
i) To evaluate and compare the nitrate adsorption capacities of raw Salix alba leaf powder (SAL) and acid-activated Salix alba leaves (ASAL);
ii) To investigate the adsorption kinetics and isotherm behavior;
iii) To study the effect of co-existing ions on nitrate adsorption.
All experiments were conducted at neutral pH to assess the practical applicability of the adsorbent in water treatment under natural conditions.
MATERIALS AND METHODS
Preparation of Adsorbent
Fresh Salix alba leaves were collected from Kargil, UT Ladakh during the months of July and August. The leaves were first rinsed with tap water followed by deionised water to eliminate any adhering dirt or impurities. After cleaning, the leaves were chopped into small pieces and oven-dried at 100 °C for 24 hours. The dried leaves were then ground into a fine powder using a mortar and pestle. The resulting powder was sieved to obtain a uniform particle size of 85 mesh and stored in a desiccator until further use.
Modification of Adsorbent
To modify the adsorbent, 10 grams of the leaf powder was soaked in 1 N hydrochloric acid (HCl) solution for 3 hours. After soaking, the material was separated and thoroughly washed with deionised water until a neutral pH was attained. The acid-treated powder was then dried in an oven at 60 °C for 24 hours. A schematic representation of the adsorbent preparation process is shown in Figure 1.

Fig. 1. Representation of preparation of sorbent
Chemicals
Analytical grade chemicals and reagents were used. Synthetic water was prepared by dissolving potassium nitrate in distil water to obtain a concentration of 100mg/L. The pH of the solution was adjusted to neutral using dilute solution of 0.1N HCl and 0.1N NaOH. The effect of co-ions on the adsorption of nitrate were examined using KH2PO4, Na2SO4, and NaCl salts in distil water to obtained required concentration of phosphate, sulphate and chloride ions.
Nitrate analysis
A calibration curve of nitrate solutions with concentration ranges of 5-100 mg/L was plotted by taking the absorbance at 220 nm using spectrophotometer22. The absorbance after adsorption was recorded and concentration of nitrate was determined. 100ml of 50mg/L solution of nitrate were used during the analysis of adsorption.
Characterization of adsorbent
The characterization of the adsorbent was done through Fourier transform infrared spectrometer (Bruker Alpha II- FTIR spectrometer) in the wavelength of 4000-500 cm-1 and Zero point charge using pH meter.
Fourier transforms infrared spectroscopy (FTIR)
The presence of surface functional groups and the effect of acid and nitrate on the surface of the adsorbent were characterised using FTIR (Bruker Alpha II- FTIR spectrometer) by scanning the sorbent sample in the wave length of 4000-500cm-1.
Determination of zero point of charge (ZPC)
A series of 50ml of 0.01M NaCl solutions were taken in volumetric flasks as base electrolyte and pH of solutions were adjusted to range of 2-10 using 0.1N HCl and 0.1N NaOH solution. 1g of adsorbent was added to each flask, shaked for 3 hours and pH was measured using pH meter as pHf.A plot of change in pH (pHf– pHi) against pHi was plotted. The ZPCof the adsorbent was recorded where ∆pH is zero. pHi and pHf are initial and final pH respectively.
Batch adsorption analysis
Effect of pH
The influence of pH on the nitrate adsorption was studied using 100 ml of 50 mg/L nitrate at different pH range of 2-10 and 0.5g of adsorbent for a contact time period of 180 minutes. The pH of the solution was adjusted by using 0.1N HCl and 0.1N NaOH. The concentration of nitrate was determined using the same approach as described in nitrate analysis.
Adsorption kinetics
The adsorption kinetics of unmodified (SAL) and acid modified (ASAL) Salix Alba leaves powder were conducted by adding 0.5 g adsorbent to a 100 ml solution of 50 mg/L nitrate solution at neutral pH and agitated the solution at the speed of 120 rpm at different intervals of time 10, 30, 50, 70, 90, 120, 140, 160, 180 minutes. At each time interval, the adsorbent was separated using whattman filter paper and concentration of nitrate in the filtrate were determined as per the procedure outlined in nitrate analysis section. The following formula 1 and 2 were used to compute the amount of nitrate adsorbed per gram of adsorbent (mg/g) and removal percentage of nitrate18.

Where, Ci is the initial concentration of nitrate solution (mg/L), Ct is the nitrate concentration at a given time (mg/L), V is volume of solution, m is mass of adsorbent and Qt is the amount of nitrate adsorbed per unit mass of adsorbent (mg/g).

Where, t is time (min), K1 is therate constant (min-1) for pseudo 1st order and K2 is the rate constant (gmg-1min-1) for pseudo 2nd order kinetics and Qe is the amount of nitrate absorbed at equilibrium (mg/g)
Adsorption equilibrium
Adsorption equilibrium was determined by taking 100 ml solution of 50 mg/L nitrate concentration in a series of flasks at neutral pH and added to them different dose of adsorbents dose of 0.1, 0.3, 0.5, 0.7,1 g. The flasks were stirred at the speed of 120 rpm for around 5 hours and then the supernatant liquid was separated using whattman filter paper. The concentration of nitrate in the filtrate was determined using the same procedure described earlier. The amount of nitrate at equilibrium was calculated using the following formula.

Qe is the amount of nitrate adsorbed per gram of adsorbent (mg/g) at equilibrium, and Ce is equilibrium concentration of nitrate (mg/L).The experimental data were simulated and applied to fit Langmuir isotherm and Freundlich isotherm model.

Qmax is Langmuir maximum amount of nitrate adsorbed (mg/g) and KL is Langmuir constant (L/mg). RL is dimensionless quantity of Langmuir isotherm Parameter and . RL gives the nature of adsorption and the relation of RL with the isotherm is depicted in table 119.
Table 1. Relation of RL with isotherm
| RL Type of isotherm |
| RL > 1 Unfavourable RL = 1 Linear 0 < RL < 1 Favourable RL = 0 Irreversible |
Freundlich model:
7)
KF is KF (mg/g (L/mg) 1/n) and n in the above equation are Freundlich constants. The favourability and sorption affinity in term of Freundlich isotherm can be elucidated by the values of (1< n <10) and(Kf >1)21.
Effect of co-ions
The effects of co-ions on the adsorption of nitrate were studied by adding Sulphate, Phosphate and Chloride of varying concentrations like 5, 10, 15, 20 mg/L in to 50 mg/L of nitrate solution and 0.5g of adsorbent at neutral pH. These co-ions were chosen since these are common ions found in all source of water7. The approach for determining nitrate concentration was followed as describe in nitrate analysis.
Data processing
MS Excel 2010 was used to simulate and compute the various parameters and draw the figures involved in adsorption kinetics and isotherms.
RESULT AND DISCUSSION
Characterization of adsorbent with FTIR
The IR spectra of pure Salix Alba leaves powder (SAL), acid treated, and nitrate loaded are illustrated in figure 2. The spectra of pure sample indicated the presence of many functional groups on the surface. The prominent peaks in the range of 2838–2843 cm-1 is due to stretching vibration of methylene in long-chain saturated alkanes (-CH2-), while the peaks around 1690-1700 cm−1 indicates stretching vibration of amide group and 1540–1640 cm−1 corresponds the existence of carbonyl groups (C=O) and carboxylic acid24. The peak around 1600 cm-1 also ascribed to C=C stretching of aromatic component and the peak at 1457 cm−1 and 1376 cm−1 are assigned to –CH2 and –CH3 bending vibrations, respectively25. The peak observed at around 1680-1600 cm-1 represents the presence of unsaturated aromatic C = C and carbonyl group. The broad peak around 3200 cm-1 is the presence of –OH group. However on acid treatment some prominent peaks around 2300 cm-1, 1650 cm-1 and 1000 cm-1 have appeared in the spectra, which indicated an interaction of acid with the surface functional groups such as carboxylic acid, alcohol, ester and ether on the surface. On loaded with nitrate a prominent peak appeared at 2300 cm-1 and 1500cm-1 indicated the presence of C=N=O and NO2 adsorbed at the surface of the adsorbent.
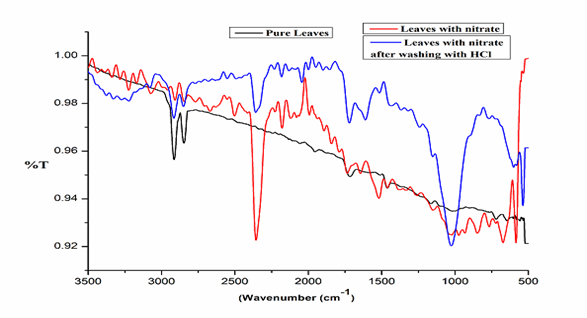
Fig. 2. FTIR Spectra of pure, acid treated and nitrate loaded sorbents
Characterization with Zero point of Charge (ZPC)
The adsorption of nitrate depends on the zero point of charge of the adsorbent. When pH of solution is greater than zero point charge, the surface of the adsorbent is negatively charged and favours the adsorption of cations and vice versa21. The zero point charge of unmodified and acid modified sorbent were recorded to 4.6 and 8.4 respectively clearly exhibited a shift toward higher pH. The adsorption of pure sorbent showed lesser adsorption capacity at neutral pH due to lower ZPC. The acid activation shifted the zero point of charge of adsorbent from 4.6 to 8.4 indicated that the modified adsorbent adsorb nitrate up to pH 8.4. The modified adsorbed showed an enhanced adsorption than the unmodified adsorbent at neutral pH. Moreover both unmodified as well as the activated sorbent showed good adsorption of nitrate at lower pH (pH 3).
Batch Adsorption analysis
Effect of pH
The pH of a solution is an essential factor. At pH 3 both unmodified (SAL) and modified Salix Alba leaves (ASAL) showed maximum adsorption toward nitrate. When pH changed from 2 to 8 the removal efficiency of unmodified Salix Alba leaves powder (SAL) decreased sharply from 85.7 to 4.7 %. However the nitrate removal efficiency of modified Salix Alba leaves powder (ASAL) decreased gradually from 74.6% to 32%. The influence of pH on the adsorption of nitrate is illustrated in figure 3. At lower pH, protonation of sorbent take place causing the surface positively charged and adsorption increased, whereas de-protonation occurred at higher pH making the surface of the adsorbent negatively charged resulting a lowered adsorption. Furthermore, at higher pH, competition between nitrate and hydroxide ion also reduced adsorption14. The acid activation increased the zero point charge of the adsorbent and as the result modified sorbent showed far better adsorption efficiency than unmodified sorbent at neutral pH.
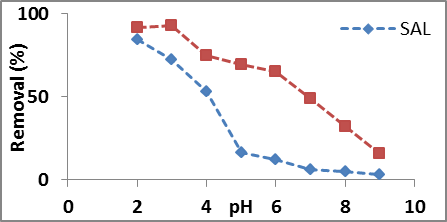
Fig 3. Effect of pH
Adsorption Equilibrium
The adsorption equilibrium isotherm was determined by using 50 mg/L nitrate solution and different dose of adsorbent of 0.1, 0.3, 0.5, 0.7,1 g. The modified Salix Alba leaves (ASAL) showed enhanced adsorption than the unmodified adsorbent at neutral pH due to induced surface positive charge by the acid. Furthermore the figure 4 illustrated that adsorption efficiency increased with the dosage of the adsorbent, since the active sites of the adsorbent increases with the dosage of the adsorbent.

Fig. 4. Effect of adsorbent dosage (g)
The experimental data of modified sorbent was simulated using MS Excel and applied to fit Langmuir and Freundlich isotherm models which are illustrated in figure 5 and figure 6.
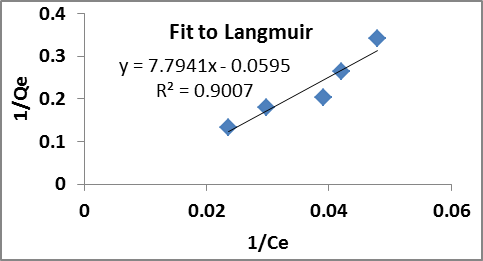
Fig. 5. fit to Langmuir isotherm model
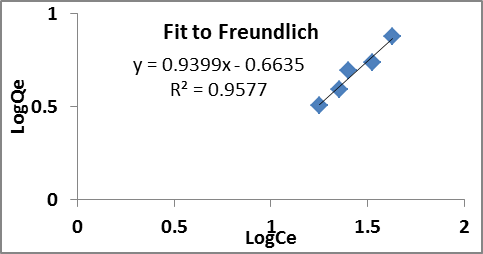
Fig. 6. Fit to Freundlich isotherm model
The Langmuir maximum adsorption capacity (Qmax) of modified sorbent come out to be 16.806 mg/g and the relevant parameters of Langmuir and Freundlich isotherm is illustrated in Table 2. The coefficient of determination (R2) for the Langmuir and Freundlich isotherm models were found to be 0.9007 and 0.9577 respectively with a better compliance to Freundlich isotherm model. The value of n and Kf favoured greater sorption affinity and compliance of Freundlich isotherm implies justifies multilayer adsorption behaviours on heterogeneous surface.
Table 2. Adsorption isotherm models and relevant parameters
| Adsorbents Langmuir isotherm Freundlich isotherm Qmax (mg/l) KL RL R2 n Kf R2 |
| ASAL 16.806 0.0076 0.723 0.9007 1.064 1.941 0.9577 |
Adsorption Kinetics
The rate of adsorption of nitrate on to the modified (ASAL) adsorbent was determined for contact time of 10-180 minute at neutral pH using 50mg/L solution of nitrate. The adsorption of unmodified sorbent (SAL) showed very less adsorption, however with the passage of time the adsorption of nitrate by modified sorbent (ASAL) increases and reached to maximum after contact time of 140 minute which is illustrated in figure 7.

Fig. 7. Effect of contact time
The experimental data of modified adsorbent were applied to fit pseudo 1st order and pseudo 2nd order which are presented in figure 8 and 9 and later model provided relatively better fit to the experimental data. Therefore the adsorption of nitrate on to the adsorbent probably involves chemisorption. The adsorption rate is influenced by the interaction of adsorption sites on the adsorbent surface with the adsorbate throughout the adsorption process i,e the adsorption behaviour justifies the presence of chemical mechanism throughout the process. The relevant parameters of the kinetics model are illustrated in table 3.

Fig. 8. Fit to Pseudo 1st order Kinetic model
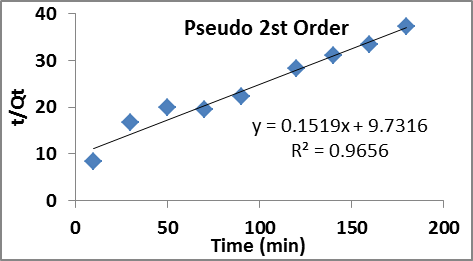
Fig. 9. Fit to pseudo 2nd order kinetic model.
Table 3. Adsorption isotherm models and relevant parameters
| Adsorbents Pseudo 1st order kinetic Pseudo 2nd order kinetic Qecal (mg/g) K1 R2 Qe exp(mg/g) Qecal(mg/g) K2 R2 |
| ASAL 6.627 0.0236 0.9391 4.89 6.583 0.00237 0.9656 |
Effect of co-ions
The adsorption of nitrate on to the adsorbent at neutral pH was studied with and without the presence of co-ions, such as sulphate, Phosphate and Chloride. The adsorption of nitrate decreased much faster in presence of sulphate. However in presence of phosphate and chloride the adsorption of nitrate decreased moderately as illustrated in figure 10. Since phosphate predominantly exist in uni-valent (H2PO4–) ion and some fraction is in bi-valent (HPO42-) ion at pH equal to or less than neutral pH. This resulted a comparatively weaker interaction of phosphate with the adsorbent. Further the adsorption efficiency decreases with increasing the concentration of co-ions from 48.9% to 3%, 12% and 14% in the presence of 20 mg/L sulphate, chloride and phosphate respectively.
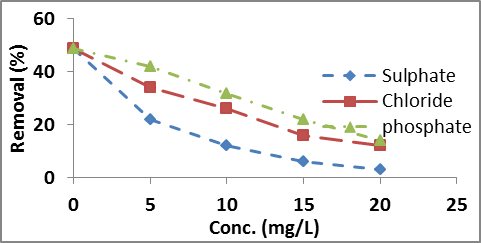
Fig. 10. Effect of co-ions on nitrate removal at different conc. (mg/L)
CONCLUSION
The experimental findings demonstrated that acid-activated Salix alba leaves are highly effective in removing nitrate from water, even at a neutral pH. Under typical water conditions, the activated Salix alba leaves exhibited significantly higher adsorption efficiency compared to the unmodified leaf powder. Although both forms of the adsorbent achieved their maximum sorption capacity at pH 3, the Langmuir model estimated the maximum adsorption capacity of the modified adsorbent at neutral pH to be 16.806 mg/g—substantially higher than that of the unmodified counterpart.
The adsorption performance of both unmodified and modified sorbents was influenced by various operational conditions and inherent properties of the adsorbents. As such, future investigations should explore the scalability and long-term performance of these materials under real-world environmental conditions, with a focus on optimizing the sorbent for diverse applications.
Kinetic and equilibrium studies suggested that the adsorption mechanism involves multiple processes, including surface interaction, diffusion, electrostatic attraction, and ion exchange. The presence of co-ions with higher negative charges significantly interfered with nitrate adsorption, leading to a noticeable decrease in removal efficiency.
Salix alba leaves are commonly used as livestock fodder in the study region, and converting them into adsorbents requires minimal capital investment. Moreover, the spent sorbents can be repurposed as nitrogen-rich fertilizers for agricultural applications. Thus, Salix alba leaves present a promising, low-cost material for pollutant removal, contributing to both environmental cleanup and sustainable agricultural waste management.
Conflict of interest
The authors declare no conflict of interest with the publication of this article.
REFERENCES
- Babuji, P.; Thirumalaisamy, S.; Duraisamy, K.; & Periyasamy, G., Water, 2023, 15(14), 2532.
- Fewtrell, L., Environ. Health Perspect., 2004, 112(14), 1371-1374.
- BIS 10500, 2012.
- WHO, 2011. Guidelines for Drinking-water Quality Fourth Ed.
- Matei, A.; Racoviteanu, G., Environ. Earth Sci., 2021, 664(1), 012024.
- Mohsenipour, M.; Shahid, S.; Ebrahimi, K., Asian J. Chem, 2014, 26(23).
- Kalaruban, M.; Loganathan, P.; Shim, W. G.; Kandasamy, J.; Ngo, H. H.; Vigneswaran, S., Sci. Total Environ, 2016, 565, 503-510.
- Bhatnagar, A.; Kumar, E.; Sillanpää, M., Chem. Eng. J., 2010, 163(3), 317-323.
- Gaikwad, R. W.; Warade, A. R., J. water resour. hydraul. eng., 2014, 3, 74-80.
- Shukla, S.; Saxena, A., Curr. Sci., 2020, 118(6), 883-891.
- Zhou, Z.; Ansems, N.; Torfs, P., International ground water assessment centre, internship report, 2015, 20, 4.
- Yacouba, Z.; Kouassi, K.; Koutouan, E.; Sombo, A.; Ekou, T.; Ekou, L., Am. J. Phys. Chem., 2021, 10, 59-66.
- Kalaruban, M.; Loganathan, P.; Shim, W.; Kandasamy, J.; Naidu, G.; Nguyen, T. V.; Vigneswaran, S., Sep. Purif. Technol., 2016, 158, 62-70.
- Hafshejani, L. D.; Hooshmand, A.; Naseri, A. A.; Mohammadi, A. S.; Abbasi, F.; Bhatnagar, A., Eco. Eng., 2016, 95, 101-111.
- Sardar, M.; Manna, M.; Maharana, M.; Sujit, Sen S., Green Adsorbents to Remove Metals, Dyes and Boron from Polluted Water, Chapter, 2020, 15, 377-403.
- Öztürk, N.; Bektaş, T. E., J. Hazard. Mater., 2004, 112(1), 155-162.
- Liu, G.; Dai, Z.; Liu, X.; Dahlgren, R. A.; Xu, J., Carbon Res., 2022, 1(1), 24.
- Kuang, P.; Cui, Y.; Zhang, Z.; Ma, K.; Zhang, W.; Zhao, K.; Zhang, X., Processes, 2023, 11(6), 1740.
- Fiaz, R.; Hafeez, M.; Mahmood, R., J. water reuse desalin., 2020, 10(1), 70-81.
- Gemici, B. T.; Ozel, H. U.; Ozel, H. B., Environ. Technol. Innov., 2021, 22, 101501.
- Hashem, A.; Aniagor, C. O.; Morsy, O. M.; Abou-Okeil, A.; Aly, A. A., Biomass Convers. Biorefin., 2022, 1-14.
- Reddy, C. A.; Prashanthi, N.; Babu, P. H.; Mahale, J. S., Development, 2015, 2(10), 94-99.
- Ahmad Khan, F.; Dar, B. A.; Farooqui, M., Int. J. Phytoremediation, 2023, 25(5), 646-657.
- Zhang, Z.; Zhou C.; Yang J.; Yan B.; Liu J.; Wang S.; Li Q.; Zhou M., Sustainability, 2022, 14(7), 4082.
- Janu, R.; Mrlik, V.; Ribitsch, D.; Hofman, J.; Sedláček, P.; Bielská, L.; Soja, G., Carbon Resour. Convers., 2021, 4, 36-46
