Introduction:
Schiff base ligand complexes with Mn (II) ions have drawn considerable interest in bioinorganic chemistry due to their structural versatility and biological activities (1). These include antibacterial, antifungal, antioxidant, anticancer (2) and anti-inflammatory properties. It is widely acknowledged for its essential role in numerous biological systems, including superoxide dismutase (3) and azide-insensitive catalase (4). Importantly, manganese in higher oxidation states is critical for the water-to-O₂ oxidation process in photosynthesis (5,6). N, O donor ligands are implicated in Manganese biological systems (7). Manganese (II) complexes containing N2O2 donor sets have been reported earlier (8,9). These complexes exhibit high spin characteristics, although some reported magnetic moments are lower than the spin-only value predicated for the S=5/2 state (8,10). Considering this, we were inspired to synthesize Mn (II) complexes using Schiff base ligand, a novel nitrogen and oxygen donor.
Experimental:
Materials and Methods:
All chemicals (AR grade) were used as received, except for Mn (II) acetate, which was recrystallized from hot acetic acid. All reagents used were analytical grade, and spectroscopic-grade solvents were employed for spectral studies.
Synthesis of Ligands:
Preparation of Ligands, bis (2,5-dihydroxy acetophenone) ethylenediamine [DAen] and bis (2,5-dihydroxy acetophenone) propylene diamine [DApn]:
The Fries rearrangement of hydroquinone diacetate produced 2,5-dihydroxyacetophenone (1). Schiff bases were synthesized by refluxing a 2:1 mixture 2,5-dihydroxyacetophenone with either ethylenediamine or 1,3-propylenediamine in ethanol for one hour. After refluxing, the hot solution was filtered, and cooling it to around 40°C led to the formation of an orange precipitate (Scheme 1). The compositions of both ligands were then analyzed. For DAen (C18H20N2O4), the results were: C, 65.39% (theoretical: 65.85%); N, 9.03% (theoretical: 8.53%); H, 6.76% (theoretical: 6.09%); λmax (nm): 354, 274, 224. For DApn (C19H22N2O4), the results were: C, 66.40% (theoretical: 66.6%); N, 8.0% (theoretical: 8.18%); H, 6.66% (theoretical: 6.43%); λmax (nm): 346, 276, 221.5.
Synthesis of Metal Complexes:
[Mn (L) Cl2.2H2O] (L=DAen; DApn):
The ligand (L) (1mmol) was dissolved in 10 mL of methanol, then the mixture was mixed with a MnCl₂ solution (1.2 mmol, 238 mg) in 5.0 mL of methanol. For two hours, the resultant solution was stirred constantly at room temperature. The produced orange products were then dried on P₄O₁₀ after being cleaned with methanol as well as ethanol.
[Mn (L) (OAc)2. nH2O] [L=Daen, DApn; n=0 (DAen; n=3 (DApn)]:
In 10 mL of methanol, ligand (L) (1mmol) was initially suspended. An Mn (OAc)₂.4H₂O (1.2 mmol, 294 mg) solution dissolved in 5.0mL of methanol was poured into this suspension. For two hours, the mixture was agitated at room temperature, resulting in the formation of deep orange precipitates. The products were subsequently dried over P₄O₁₀ after being cleaned with methanol and diethyl ether.
[Mn (L) (SCN)2.2H2O] (L=DAen; DApn):
A saturated solution of KSCN was employed to treat a MnCl₂ (1.2 mmol, 238 mg) solution in methanol until precipitation of KCl ceased. The mixture was then centrifuged, and the resulting filtrate was combined with ligand suspended (1 mmol) in 5 mL of methanol. For two hours, this combined mixture was agitated at room temperature. After filtering & washing with methanol as well as diethyl ether, the orange products were dried on P₄O₁₀.
Analytical and Spectral Measurements:
C, H, and N analyses were performed through microanalytical methods at Delhi University (USIC), Delhi-7. The manganese content was measured using Atomic Absorption Spectroscopy (AAS) (see Table 1). A Perkin-Elmer 554 Spectrometer was employed to record electronic spectra. A Perkin-Elmer R-32 spectrometer operating at 90MHz was employed to acquire 1H NMR spectra. Utilizing a Shimadzu IR-435 spectrometer with Nujol mull infrared (IR) spectra were collected. EPR spectra were measured at X-band frequency. A Cahn 2000 Magnetic Balance was employed to measure magnetic susceptibility, and Pascal’s constants were utilized to apply diamagnetic corrections. The structure of the Mn (II) complexes was illustrated using ACS Chemsketch software.
Table 1: Elemental Analysis of Mn (II) Complexes:
| Complex | % C(calc) | %N(calc) | %H(calc) | %Mn(calc) |
| [Mn(DAen)Cl2].2H2O | 43.8(44.1) | 4.4(4.9) | 6.2(5.7) | 11.5(11.2) |
| [Mn(DAen)(OAc)2] | 52.2(52.7) | 5.0(5.2) | 6.0(5.6) | 12.0(11.0) |
| [Mn(DAen)(SCN)2] | 48.5(48.1) | 3.9(4.0) | 11.6(11.2) | 10.9(11.0) |
| [Mn(DApn)Cl2].2H2O | 45.7(45.2) | 4.7(5.2) | 6.0(5.6) | 11.4(10.9) |
| [Mn(DApn)(OAc)2].3H2O | 48.9(48.5) | 5.4(6.0) | 5.4(4.9) | 10.2(9.7) |
| [Mn(DApn)(SCN)2] | 49.5(49.1) | 4.2(4.3) | 10.7(10.9) | 11.0(10.7) |
Results and Discussion:
Electronic Spectroscopy:
The ligands, DAen and DApn are found to be soluble in methanol in the presence of sodium hydroxide or DMSO or DMF. Their absorption spectra in MeOH/NaOH have three prominent bands at ʎmax around 224, 275, and 355 nm. While in DMSO the ligands show a single band around 360 nm (due to cut-off at 280 nm in DMSO). The faint orange colour observed is attributed to a band around 360 nm, resulting from an n-π* transition involving the lone-pair electrons of nitrogen. This is similar to the behaviour seen in the N, N’-di benzylidene-ethylenediamine chelate (11). In the UV region, the high-energy bands exhibit narrowing and a bathochromic shift of approximately 10 nm in the N, N’-bis (o-hydroxy benzylidene) ethylenediamine chelate. These effects are likely due to hydrogen bonding, which enhances molecular rigidity and increases conjugation between the C=N groups and the benzene rings, bringing them closer to a coplanar arrangement (11). The band around 274 nm may be associated with π-π* transition of >C=N group, while π→π* benzenoid vibrations produce a band near 220 nm, which was observed at 224 nm in the current ligands DAen and DApn.
The complexes exhibit absorption spectra characteristic of Schiff base ligands. The band around 360 nm in the free ligand shifts by 10-15 cm⁻¹ upon complexation with the manganese (II) centre, indicating ligand binding to the metal. Due to the limited solubility of these Mn (II) complexes, extinction coefficients are not provided (see Table 2). In the case of high-spin manganese (II), no d-d transitions are observed, as these transitions are both spin-forbidden and Laporte-forbidden.
IR Spectroscopy:
IR spectra of the ligand and manganese (II) complexes were recorded in Nujol mull. By earlier assignments for Schiff bases, a noticeable band at about 1600cm⁻¹ in the free ligands (DAen and DApn) is linked to the stretching mode of v(C=N) (12). This band shifts downwards by 20-30 cm⁻¹ in the complexes, suggesting that the imine nitrogen atoms are coordinating directly with the Mn (II) ion. Additionally, a peak near 1380 cm⁻¹, corresponding to ν(C-N) in the free ligand, shifts down by 10-20 cm⁻¹ in the complexes. The band assigned to ν(C-O), in the free ligand at 1260 cm⁻¹, shifts 10-15 cm⁻¹ to higher frequency upon coordination. Peaks in the range of 1200-960 cm⁻¹ are attributed to in-plane deformation vibrations of the aromatic rings, while those around 820-780 cm⁻¹ are associated with aromatic C-H rocking. Furthermore, the peak around 2900 cm⁻¹, attributed to the phenolic –OH group, shifts approximately 50 cm⁻¹ following coordination, indicating that the –OH group remains protonated.
In the [Mn (DAen)(OAc)₂] complexes, novel absorption bands are noted at 1555 & 1370cm⁻¹, along with bands at 1500 and 1330cm⁻¹ in the related complexes. The unidentate binding mode of the acetate group that is attached to the metal centre is indicated by these features. For the [Mn (DAen)(SCN)₂] & [Mn (DApn)(OAc)₂] complexes, the thiocyanate-related bands appear at 2000 cm⁻¹ and 2050 cm⁻¹, respectively. These bands are likely indicative of a nitrogen-bonded thiocyanate group, as stretching frequencies for N-bonded complexes typically fall below 2100 cm⁻¹, contrasting with the higher frequencies observed in five-coordinate complexes, which are generally above 2100 cm⁻¹.
Magnetic Susceptibility Measurements:
A CAHN 2000 Balance was employed to measure the manganese complexes’ magnetic susceptibility in the solid state at 304 K. Employing Pascal’s constants, diamagnetic adjustments were computed for every complex and applied to the experimental susceptibility measurements. Table 3 provides magnetic moment data. The magnetic susceptibility analysis outcomes for every complex are consistent with the formulations proposed based on their EPR data.
The room temperature magnetic moment values fall within the typical range observed for other d⁵ Mn (II) complexes. This aligns with the Mn (II) ion’s ground state, which, in a near-octahedral field, exhibits a 6A₁g ground state that lacks orbital angular momentum. However, the values are slightly lower than expected, which suggests that this reduction in magnetic moment may be due to weak intermolecular exchange interactions.
Table 2: Observed optical data for Mn (II) Complexes in DMSO:
| Complex | λmax |
| [Mn(DAen)Cl2].2H2O | 360 |
| [Mn(DAen)(OAc)2] | 352 |
| [Mn(DAen)(SCN)2] | 342 |
| [Mn(DApn)Cl2].2H2O | 354 |
| [Mn(DApn)(OAc)2].3H2O | 352 |
| [Mn(DApn)(SCN)2] | 346 |
Table 3: Magnetic data for Mn (II) Complexes at 300K:
| Complex | µeff/atom (B.M.) |
| [Mn(DAen)Cl2].2H2O | 5.54 |
| [Mn(DAen)(OAc)2] | 5.28 |
| [Mn(DAen)(SCN)2] | 5.50 |
| [Mn(DApn)Cl2].2H2O | 5.23 |
| [Mn(DApn)(OAc)2].3H2O | 5.72 |
| [Mn(DApn)(SCN)2] | 5.85 |
EPR Spectroscopy:
Figures 1-6 illustrate X-band EPR spectra of manganese (II) complexes, which are diluted in their respective ligands. A single isotropic line is observed at g = 2.0, with line widths ranging from 325 to 500 gauss. Notably, the absence of additional signals in the 5000 Gauss scan indicates a lack of significant zero-field splitting, which is often expected for non-cubic manganese (II) complexes. This straightforward spectrum, devoid of hyperfine structure, suggests that the Mn(II) complex is influenced by magnetic interactions with neighbouring atoms (13).
The simplicity of the observed EPR spectrum may also indicate a highly symmetrical environment around the manganese ion, potentially reflecting the coordination geometry and ligand field strength. Variations in line width can be attributed to differences in the electronic environment and local symmetry of the manganese centres in the complexes.
Additionally, the temperature dependence of EPR signals could provide insights into dynamic processes within the complexes. Such investigations might reveal temperature-related changes in the magnetic interactions or alterations in the ligand environment that could affect the electronic states of the manganese ion. Table 4 summarizes the peak-to-peak line widths and the D parameter for the EPR signals, contributing further to our understanding of the electronic structure and behaviour of these complexes.
The D parameter for all complexes was calculated using the Korteweg and Reijen method (14), as detailed in Table 4. For those complexes exhibiting a peak-to-peak line width of 500 gauss or greater, it is inferred that the observed resonance corresponds to a single manganese (II) ion rather than an exchange-coupled complex. D values obtained range from 0.024-0.062cm⁻¹ (see Table 4), indicating minimal zero-field splitting. The primary resonance around g=2 suggests that the non-cubic crystalline electric fields are weak, implying that the Mn²⁺ ion retains nearly cubic symmetry.
EPR spectra for the manganese (II) complexes were recorded at both 140 K and room temperature. Upon comparison, no significant differences were noted, indicating a lack of substantial temperature dependence in the observed EPR signals. This analysis aimed to identify any strong variations in line width, which are typically associated with strongly exchange-coupled systems that would exhibit noticeable line width changes at different temperatures.
The consistent EPR results across these temperatures suggest that the magnetic interactions within the complexes remain stable and are not significantly influenced by thermal fluctuations. This stability may also reflect the robust nature of the ligand field surrounding the manganese ions. Further investigations could explore the effects of varying ligand environments or additional external parameters on the magnetic properties and EPR characteristics of these complexes.
Table 4: EPR data for the Mn (II) Complexes
| Complex | RT | LT | ||
| Line Width | D1 | Line Width | D2 | |
| [Mn(DAen)Cl2].2H2O | 500 | 0.026 | 500 | 0.026 |
| [Mn(DAen)(OAc)2] | 450 | 0.024 | 475 | 0.025 |
| [Mn(DAen)(SCN)2] | 465 | 0.024 | 500 | 0.026 |
| [Mn(DApn)Cl2].2H2O | 415 | 0.44 | 425 | 0.045 |
| [Mn(DApn)(OAc)2].3H2O | 325 | 0.051 | 365 | 0.057 |
| [Mn(DApn)(SCN)2] | 375 | 0.058 | 400 | 0.062 |
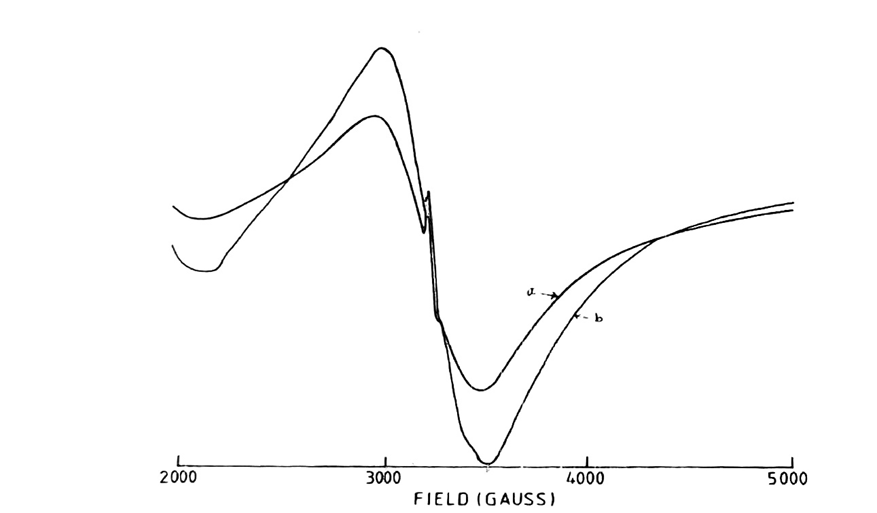
Fig. 1: 5000 Gauss scan centered at 2500G of [Mn (DAen)Cl2]. H2O in solid state.
Microwave power 10mV; frequency 9.03 GHz: (a) Modulation amplitude 2.0G; r.gain in 10×102; T=250C; (b) Modulation amplitude 6.3 G: r.gain in 4×102: T=138K

Fig. 2: 5000 Gauss scan centered at 2500G of [Mn (DAen)(OAc)2] in the solid state.
[Microwave power 10mV: frequency 9.03 GHz: Modulation amplitude 0.5G: r.gain T=250C]
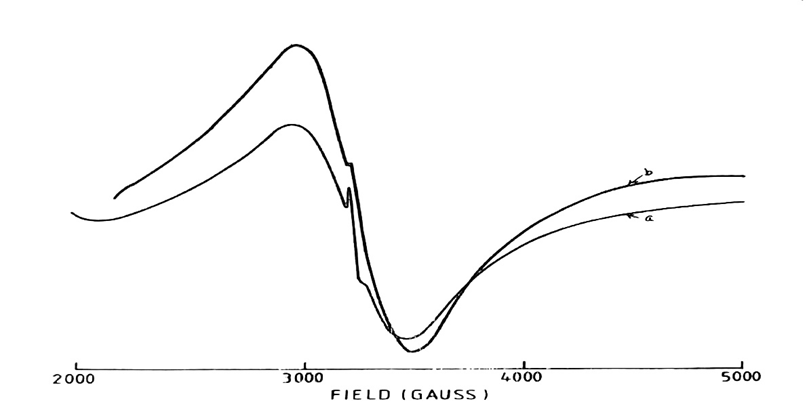
Fig. 3: 5000 Gauss scan centered at 2500G of [Mn (DAen)(SCN)2] in the solid state.
[Microwave power 10mV: frequency 9.03GHz: (a) Modulation amplitude 2.0G: r.gain in 10 x102: T=250C: (b) Modulation amplitude 0.5G: r.gain in 5×102: T=138K]
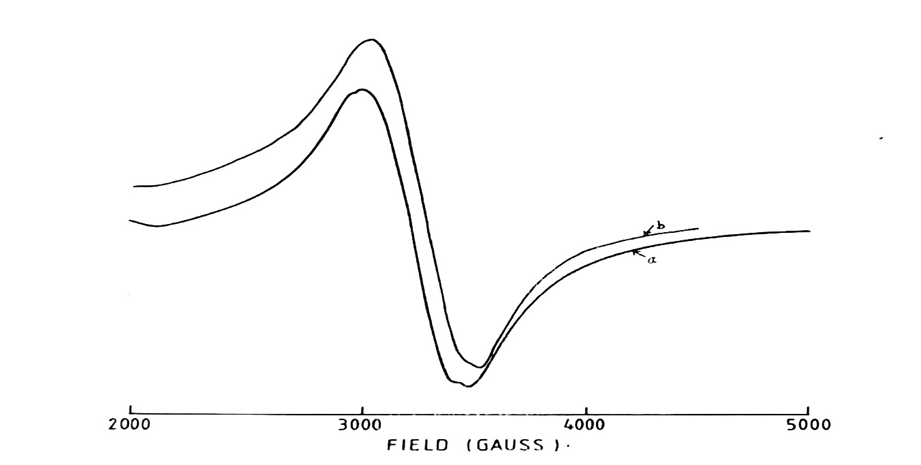
Fig. 4: 5000 Gauss scan centered at 2500G of [Mn (Dpen)Cl2]. 2H2O in the solid state.
[Microwave power 10mV: frequency 9.03GHz: (a) Modulation amplitude 2.0G: r.gain in 10 x102: T=250C: (b) Modulation amplitude 0.5G: r.gain in 3.2×102: T=138K]
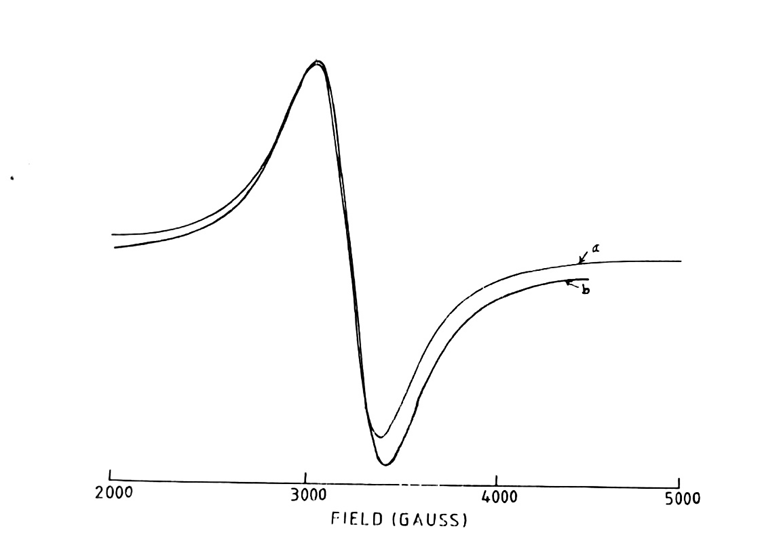
Fig. 5: 5000 Gauss scan centered at 2500G of [Mn (DApn)(OAc)2].3 H2O in the solid state.
[Microwave power 10mV: frequency 9.03GHz: Modulation amplitude 0.5G: r.gain T=250C]
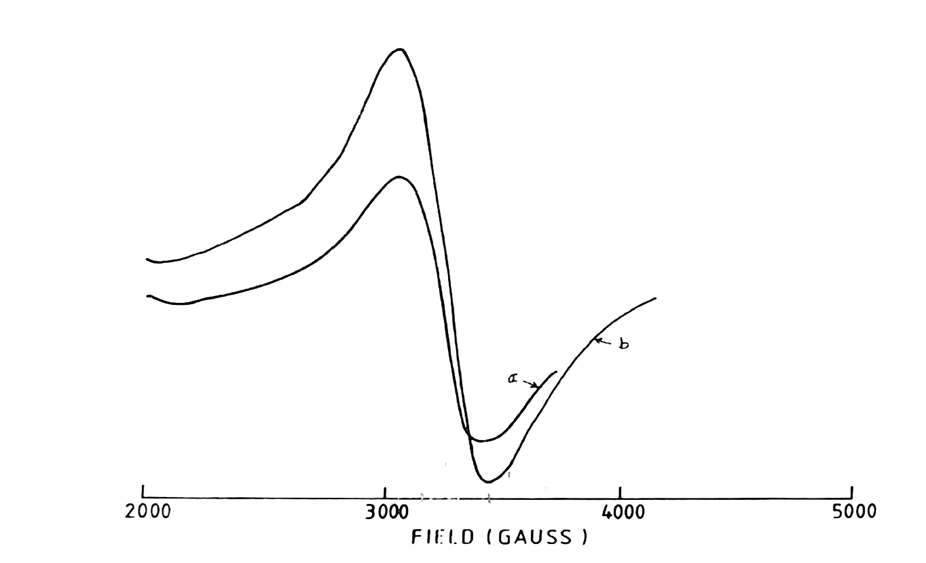
Fig. 6: 5000 Gauss scan centered at 2500G of [Mn (DApn)(SCN)2] in the solid state.
[Microwave power 10mV: frequency 9.03GHz: (a) Modulation amplitude 0.8G: r.gain in 2.5×102: T=250C: (b) Modulation amplitude 0.5G: r.gain in 10×102: T=138K
Conclusion:
Bivalent mononuclear manganese (II) complexes have been synthesized using two ligands: bis (2,5-dihydroxy acetophenone) ethylenediamine (DAen) and bis (2,5-dihydroxy acetophenone) propylenediamine (DApn). The existence of 2 anions in Mn (II) complexes, as indicated by C, H, and N analysis, manganese estimation, and IR data, suggests that the ligand binds to the manganese (II) centre in its protonated form. This binding behaviour is expected in strongly exchange-coupled systems, which typically exhibit significant line width variations. The observed quenching in magnetic moment is attributed to weak intermolecular exchange interactions. Based on these studies, tentatively proposed structures for the manganese (II) complexes are illustrated in Fig. 7.

Conflict of Interest:
Regarding the publishing of this paper, I hereby declare that I have no conflicts of interest. On behalf of all contributing authors, I, the corresponding author, hereby attest that, to the best of my knowledge and belief, the information provided in this disclosure is accurate and comprehensive.
References:
- Schiff base, from Wikipedia. https:// en. Wikipedia.org/wiki/Schiff base.
- Chang, G. L.; Li, Z.; Niu, M. J. and Wang, Su-Na, “Studies on the manganese and copper complexes derived from chiral Schiff base: synthesis, structure, cytotoxicity, and DNA/BSA interaction,” Journal of Coordination Chemistry, 2019, vol. 72, no. 14, 2422–2436
- Ludwig, M. L.; Pattridge, K. A. and Stallings, V. C., “Manganese in Metabolism and Enzyme Function” eds. Schramm, V. L. and Wedler, F. C., Academic Press, New York, 1986, Ch.21, 405
- Beyer, W. F. and Fridovitch, I. Manganese in Metabolism and Enzyme Function, eds. Schramm, V. L. and Wedler, F. C., Academic Press, New York, 1986, Ch. 12, 193
- Dismukes, G. C. Manganese in Metabolism and Enzyme Function, eds. Schramm, V. L. and Wedler, F. C., Academic Press, New York, 1986, Ch. 16, 275.
- Pecoraro, V. L., Photochem Photobiol., 1986, 48, 249.
- Mathur, P.; Crowder, M. and Dismukes, G. C. “Dimanganese complexes of a septadentate ligand. Functional analogues of the Manganese Pseudo catalase” J. Am. Chem. Soc. 1987, 109, 5227-5233.
- Poddar, S. N., and DayK. Z. Anorg. Allgem. Chem., 1964, 327, 104
- Mukherjee, A. K., and Ray, P. J Indian Chem. Soc., 1955, 32, 581
- Willis, J. B. and Mellor, D. P., J. Am. Chem. Soc., 1947, 69, 1237
- Crawford, S. M., Spectrochimica Acta, 1963, 19, 255
- Kushekar, A. and Khaolkar, D. D., Indian J. Chem., 1983, 22A, 881
- Dowsing, R. D.; Gibson, J. F.; Goodgame, D. M. L.; Goodgame, M. and Hayward, D. J., Nature, 1968, 219, 1037
- Korteweg, G. A. and Van Reijen, L. L., J. Mag. Res., 1981, 44, 159
