Introduction:
It is widely believed that Earth is a water-rich planet, with water covering approximately 71% of its surface. This vast reserve is continually recycled and reused through natural processes. One of the most remarkable aspects of this recycling is water’s inherent self-purification ability. The process by which water moves through this continuous cycle is known as the hydrological cycle, though it involves only about 1% of the total water on Earth [1–2]. Around 97% of the Earth’s water exists as saltwater in the oceans, while just 3% is freshwater—most of which is locked in polar ice caps and glaciers. Of the estimated 0.011 million kiloliters of water available on the planet, only about 33,400 kiloliters are usable for drinking, domestic, agricultural, and industrial purposes. The rest remains in oceans, polar ice, or underground. Groundwater alone accounts for approximately 29.9% of all accessible freshwater [3–4].
In daily life, the availability of water—primarily from surface and groundwater sources—has become crucial. Usable land for supplying clean water is limited, yet it is essential for drinking, agriculture, energy production, industry, transportation, and waste disposal [5]. Water can be sourced from wells, ponds, rivers, lakes, and other natural systems; however, pure, clean, and safe water is only briefly present in nature before being contaminated by environmental factors and human activity. Therefore, most water sources require treatment before they are safe for use [6–8]. Groundwater, located within soil pores and permeable geological layers, originates from precipitation, snowmelt, and seepage from water bodies like lakes and rivers [9–11].
Groundwater serves as a vital source for wells, springs, bore wells, and hand pumps. Rapid population growth and urban expansion have led to a shortage of potable water, forcing people to increasingly rely on groundwater, particularly via hand pumps, due to irregular or inadequate piped water supply. In many Indian states, over 90% of the population depends on groundwater for drinking and other daily needs [12–14]. It is also a critical source for irrigation and industrial operations. Groundwater quality, determined by its chemical composition, directly influences its suitability for various uses. Shallow, unconfined aquifers lacking protective clay layers are more prone to contamination than deeper, confined ones. Changes in nearby land use and water movement patterns can significantly alter the quality and availability of underground water [15–17].
Water pollution can be defined in multiple ways, typically referring to the buildup of harmful substances in water that pose risks to humans and animals. While natural water bodies have some capacity to dilute and detoxify pollutants, this ability depends on the volume of contaminants. A small amount of a harmful substance might have negligible environmental impact, but large quantities can be devastating. Practices such as discharging untreated sewage into soak pits have led to groundwater contamination in many areas [18–20]. Pollutants from septic tanks, seepage pits, and waste disposal sites can percolate through soil layers and enter aquifers. Additionally, transportation-related accidents and industrial waste contribute to this problem. Agricultural modernization has also played a role; overuse of nitrogen-based fertilizers leads to nitrate infiltration into groundwater. Nitrates, being water-soluble, seep through the soil and accumulate in aquifers, posing serious health risks like methemoglobinemia, especially in bottle-fed infants [21–22].
Urban areas face the highest groundwater contamination due to dense waste disposal in limited spaces. Increased human activities near water bodies can damage aquatic ecosystems and alter water’s biological and physicochemical properties. According to the World Health Organization (WHO), 30–80% of diseases in humans are linked to contaminated water. In developing countries, many people depend on shallow wells, which are especially susceptible to pollution—mainly from human actions. These sources of pollution can be grouped into four categories: municipal, industrial, agricultural, and individual. Municipal contamination includes open dumps, poorly managed landfills, and unsanitary latrines [23–25]. These sources often carry harmful pathogens and toxic substances like heavy metals that can leach into groundwater. Industrial sources include wastewater discharge, mining activities, and leaks or spills from factories. Individual actions, such as illegal dumping of waste, also play a significant role. The chemical composition of groundwater varies significantly even over short distances, largely due to differing hydrochemical processes [26–29].
To assess groundwater quality in the tribal region of Nandurbar district, the Taloda tehsil was selected for this study. Groundwater samples were collected from wells and bore wells across various villages using sterile plastic containers, with each site contributing 1.5 liters of water. These samples were immediately analyzed for various physicochemical parameters, including temperature, turbidity, pH, electrical conductivity (EC), total dissolved solids (TDS), total hardness (TH), chlorides, alkalinity, fluoride, nitrate, sulphate, calcium, and magnesium, among others.
Material and methods:
- Groundwater sampling:
The pre-monsoon (May) and post-monsoon (November) seasons of 2022 gathered water samples from nine different sampling locations of Taloda tehsil. These samples were collected respectively. Sterilized water cans with a capacity of 1.5 liters were used to collect water during the morning hours, between the hours of 8:00 and 10:00 AM. Before being carried to the laboratory in an ice box and kept at a temperature of 4 degrees Celsius, the sample code was affixed to the cans. In accordance with the established procedures (APHA 2005), the samples were taken to the laboratory for examination. Following the completion of the sample at the location, the parameters pH and DO were analyzed immediately.
- Methodology:
The samples were carefully preserved in a laboratory after collection from a number of villages in the Nandurbar District. Furthermore, numerous parameters were analyzed using standard procedures as described in the 23rd edition of APHA’s Standard procedures for the Examination of Water and Wastewater [30-32] and other relevant sources.
- Result and Discussion:
- Temperature of groundwater sample:
Provide information on the summer, monsoon, and winter temperatures of samples taken from various Taloda tehsil villages. The findings indicate that throughout in summer, rainy season, and midwinter, correspondingly, the highest temperature of the groundwater sample is 31° C, 20.7° C, and 29° C as shown in Fig.1.
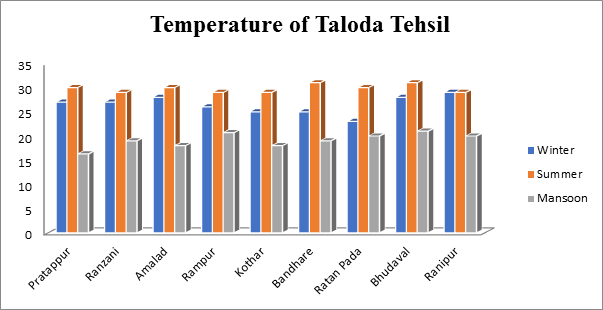
Fig.1. Temperature of groundwater sample from Taloda Tehsil
- Turbidity of groundwater sample
The turbidity values of groundwater samples taken throughout the summer, monsoon, and winter seasons diverse from 0.0 to 6.3 mg/L in the current research as shown in Fig.2. The highest amount, 6.3 mg/L, was recorded in a groundwater sample taken in the monsoon time from the village of Bhudaval.
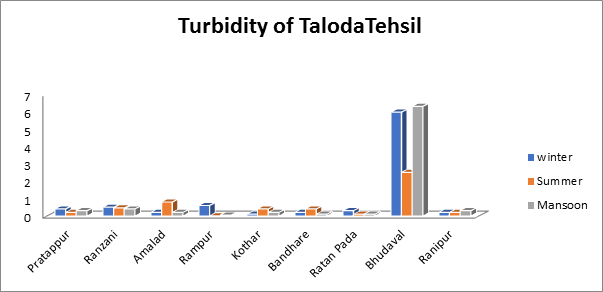
Fig.2. Turbidity of groundwater sample from Taloda Tehsil
- pH of groundwater sample:
The pH ranges of groundwater samples taken in summer, monsoon, and winter from various villages in Taloda tehsil were found to be 6.3 to 7.6, 7.4 to 7.9, and 7.3 to 7.6 over the course of the current investigation, as indicated by. The attendance of Carbonic acid salt and Acid carbonate in the water sample caused its overall pH to be measured between 6 and 8, which is the maximum pH value seen during the monsoon season. as shown in Fig.3.
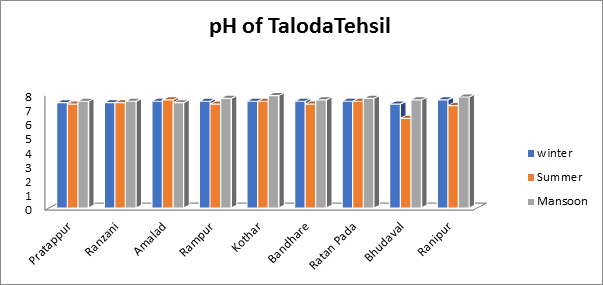
Fig.3. pH of groundwater sample from Taloda Tehsil.
- Electrical Conductance (EC) of groundwater sample:
In this investigation, demonstrate that the highest electrical conductivity values measured in summer, monsoon, and winter seasons, respectively, were 300, 231 and 400 μS/cm for water samples taken from various villages. The groundwater samples were found to have overall electrical conductivity values between 200 and 400 μS/cm as shown in Fig.4. All of the water samples had electrical conductivity values that were below the BIS-recommended ideal level.
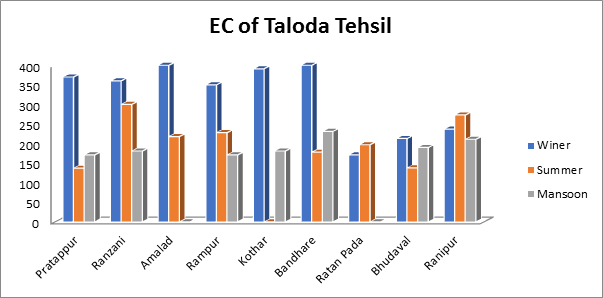
Fig.4. Electrical Conductance (EC) of groundwater sample from Taloda Tehsil.
- Total Dissolved Solids of groundwater sample:
The highest TDS value of the groundwater sample used in this study for the several villages in Taloda Tehsil is 911, 830, and 712 mg/L in the summer, rainy, and winter periods, correspondingly. Taloda tehsil’s ground water is unfit for human consumption without any purification treatment, since the TDS readings for every season were higher than allowed as shown in Fig.5. This is because the disintegration of soil particles is what causes the concentration of TDS in ground water to rise.
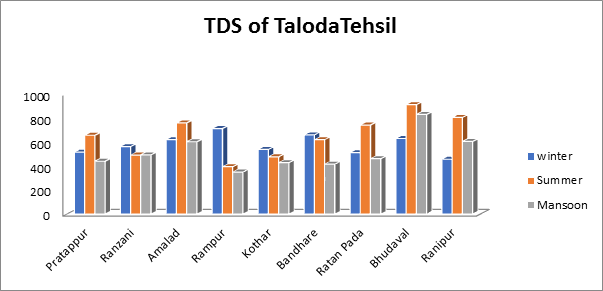
Fig.5. Total Dissolved Solids of groundwater sample
- Alkalinity of groundwater sample:
For the alkalinity levels in the current study, the range was minimum 84.5 mg/L to maximum 253 mg/L in the summer and minimum 83 mg/L to maximum 237 mg/L in the monsoon season. Alkalinity readings throughout the winter months varied from 92 mg/L to a max of 260 mg/L as shown in Fig.6. A flavor that is too strong may be produced by exceeding the 200 mg/L BIS permissible limit for total alkalinity.
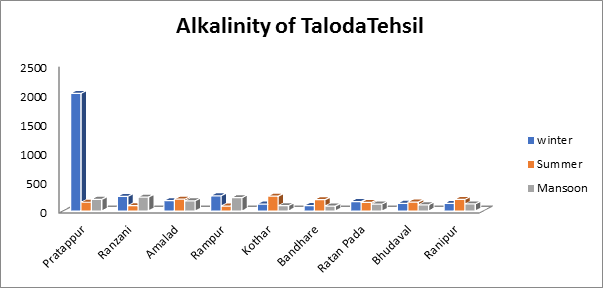
Fig.6. Alkalinity of groundwater sample of Taloda tehsil.
- Chloride of groundwater sample:
Chloride readings in the current study varied by season, from a low of 98 mg/L to a maximum of 180 mg/L in the summer to a minimum of 30 mg/L to a maximum of 282 mg/L in the monsoon. The chloride readings varied from 46 mg/L to a maximum of 326 mg/L over the winter as shown in Fig.7. Additionally, the findings show that the amount of chloride in Taloda Tehsil’s groundwater is within allowable bounds
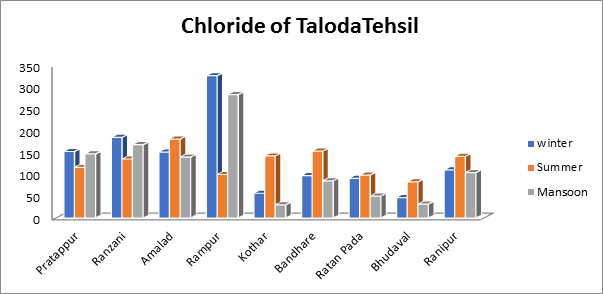
Fig.7. Chloride of groundwater sample from Taloda Tehsil
- Fluoride of groundwater sample:
The current study found that fluoride levels varied according to the season, with minimum levels of 0.0 mg/L during the summer and maximum levels of 0.2 mg/L during the monsoon. Fluoride levels in the winter varied from 0 mg/1 to a high of 0.3 mg/L. as seen in Fig.8. Additionally, the findings show that Taloda tehsil’s groundwater contains fluoride within allowable bounds
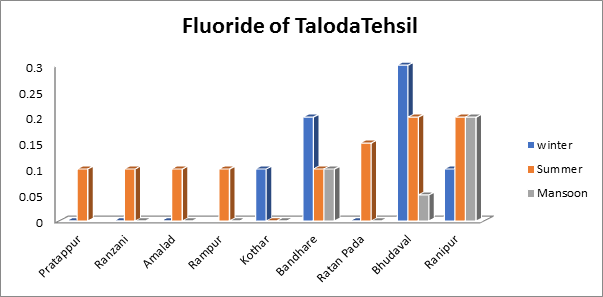
Fig.8. Fluoride of groundwater sample from Taloda Tehsil
- Nitrate of groundwater sample:
The current analysis revealed that the nitrate levels diverse from 8.5 mg/L to a high of 27.2 mg/L during the monsoon season and from a min of 23.5 mg/L to a max of 51.5 mg/L during the summer as seen in Fig.9. illustrate the range of nitrate values observed over the winter season. As opposed to the hot and monsoon periods, the grades showed that the nitrate content increased throughout the winter.
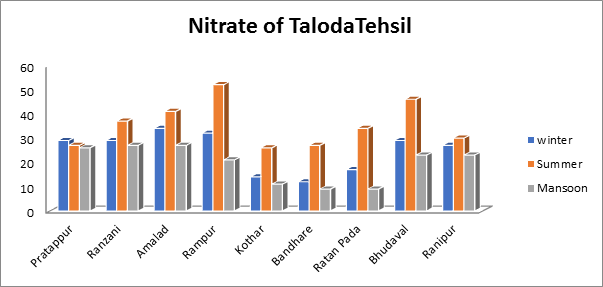
Fig.9. Nitrate of groundwater sample from Taloda Tehsil
- Sulphate of groundwater sample:
The range of Sulphates values in the current investigation was minimum 10.7 mg/L to maximum 37 mg/L in the summer and minimum 3.5 mg/L to maximum 25.7 mg/L in the monsoon season. Sulphate readings throughout the winter months varied from 7 mg/L to 27.7 mg/L as seen in Fig.10. Sulfate can be present in quantities up to 400 mg/L. The sulfate levels in the current study are all well below the guidelines for drinking water regulations, regardless of the season.
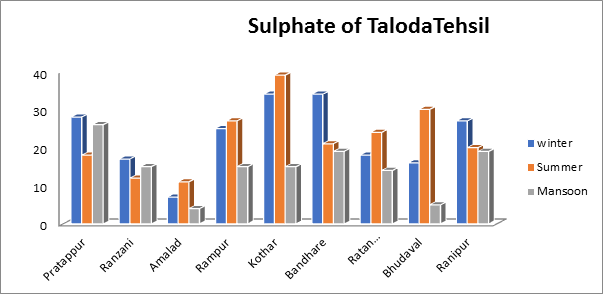
Fig.10. Sulphate of groundwater sample from Taloda Tehsil.
- Total Hardness of groundwater sample:
In the current analysis, the total hardness values varied from 117 mg/L at minimum to 462 mg/L in summer and from 140 mg/L at lowest to 326 mg/L in monsoon as seen in Fig.11. An illustrate the range of hardness values observed over the winter season. For overall hardness, a range of 600 mg/L is acceptable. All three seasons’ combined hardness levels in the current study are higher than the recommended threshold for drinking water regulations.
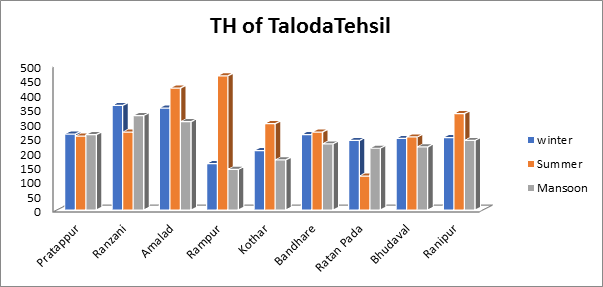
Fig.11. Total Hardness of groundwater sample from Taloda Tehsil
- Calcium of groundwater sample:
The Calcium levels in the current investigation varied from a little of 42 mg/L to a high of 98 mg/L during the summer to a min of 01 mg/L to a max. of 57 mg/L during the monsoon. According to the calcium levels varied from 17 mg/L to 81 mg/L as seen in Fig.12. A drinking water’s permissible calcium content is 200 mg/L. All of the water samples used in this investigation is under the standard drinking water acceptable level.
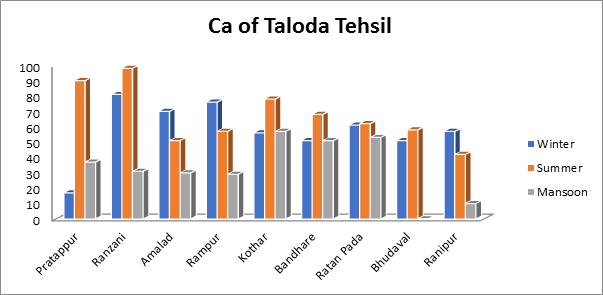
Fig.12. Calcium of groundwater sample from Taloda Tehsil.
- Magnesium of groundwater sample:
In this particular analysis, the magnesium levels varied from a minimum of 13 mg/L to a high of 42 mg/L during the summer to a minimum of 12 mg/L to a maximum of 31 mg/L during the monsoon as seen in Fig.13. According to the magnesium levels varied from 09 mg/L to 72 mg/L over the winter. Magnesium in drinking water may include up to 100 mg/L of acceptable magnesium. All of the water samples used in this investigation is safe and under permissible limit
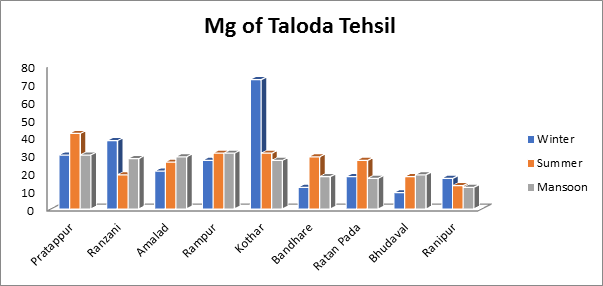
Fig.13. Magnesium of groundwater sample from Taloda Tehsil.
- Conclusion
An understanding of the fluctuations in water quality can be gained from the data obtained from laboratory tests conducted in the summer, monsoon, and winter using physico-chemical analysis of groundwater samples of Taloda tehsil. Seasonal effects on water quality are indicated by variations in the tested values of parameters like turbidity, hardness, pH, conductivity, alkalinity, sulphates, total dissolved solids (TDS), and chloride. Seasonal variations were also seen in conductivity, alkalinity, sulphates, pH, turbidity, and hardness. It is clear by comparing the tested results to the BIS allowable and desirable limits that, during some seasons, several metrics may exceed the permissible or ideal limits, raising possible issues about the quality of the water. For instance, the tested values for alkalinity and TDS during the monsoon season were higher than the BIS allowable limits, indicating the need for additional attention to the management of water quality during that season. This suggests that the water might not always be safe to drink all year round. To make sure the water is suitable for drinking; more research and quality control are required.
Conflict of interest:
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Reference:
- Abu Shmeis, R. M. (2018). Chapter One – Water Chemistry and Microbiology. In Chormey, D. S., Bakırdere, S., Turan, N. B., & Engin, G. Ö. (Eds.), Comprehensive Analytical Chemistry (Vol. 81, pp. 1-56).
- Asthana, M., Kumar, A., & Sharma, B. S. (2017) Wastewater Treatment. In Singh, R. L. (Ed.), Principles and Applications of Environmental Biotechnology for a Sustainable Future (pp. 173-232). Singapore: Springer Singapore.
- Issaka, S., & Ashraf, M. A. (2017). Impact of soil erosion and degradation on water quality: a review. Geology, Ecology, and Landscapes, 1(1), 1-11.
- Ding, L., Chen, K.-l., Cheng, S.-g., & Wang, X. (2015). Water ecological carrying capacity of urban lakes in the context of rapid urbanization: A case study of East Lake in Wuhan. Physics and Chemistry of the Earth, Parts A/B/C, 89-90, 104-113.
- Kang, P., & Xu, L. (2012). Water Environmental Carrying Capacity Assessment of an Industrial Park. Procedia Environmental Sciences, 13, 879-890.
- Richa, A., Touil, S., & Fizir, M. (2022). Recent advances in the source identification and remediation techniques of nitrate contaminated groundwater: A review. Journal of Environmental Management, 316, 115265.
- Abu Shmeis, R. M. (2018). Chapter One – Water Chemistry and Microbiology. In Chormey, D. S., Bakırdere, S., Turan, N. B., & Engin, G. Ö. (Eds.), Comprehensive Analytical Chemistry (Vol. 81, pp. 1-56). Elsevier.
- Asthana, M., Kumar, A., & Sharma, B. S. (2017). Wastewater Treatment. In Singh, R. L. (Ed.), Principles and Applications of Environmental Biotechnology for a Sustainable Future (pp. 173-232). Singapore: Springer Singapore.
- Issaka, S., & Ashraf, M. A. (2017). Impact of soil erosion and degradation on water quality: a review. Geology, Ecology, and Landscapes, 1(1), 1-11.
- Ding, L., Chen, K.-l., Cheng, S.-g., & Wang, X. (2015). Water ecological carrying capacity of urban lakes in the context of rapid urbanization: A case study of East Lake in Wuhan. Physics and Chemistry of the Earth, Parts A/B/C, 89-90, 104-113.
- Kang, P., & Xu, L. (2012). Water Environmental Carrying Capacity Assessment of an Industrial Park. Procedia Environmental Sciences, 13, 879-890.
- Richa, A., Touil, S., & Fizir, M. (2022). Recent advances in the source identification and remediation techniques of nitrate contaminated groundwater: A review. Journal of Environmental Management, 316, 115265.
- Abdelwaheb, M., Jebali, K., Dhaouadi, H., & Dridi-Dhaouadi, S. (2019). Adsorption of nitrate, phosphate, nickel and lead on soils: Risk of groundwater contamination. Ecotoxicology and Environmental Safety, 179, 182-187.
- Klay, S., Charef, A., Ayed, L., Houman, B., & Rezgui, F. (2010). Effect of irrigation with treated wastewater on geochemical properties (saltiness, C, N and heavy metals) of isohumic soils (Zaouit Sousse perimeter, Oriental Tunisia). Desalination, 253(1), 180-187.
- Zeydalinejad, N., Mahdavikia, H., Goudarzi, A., & Saeidi, S. (2023). The present challenges and policy for sustainable management of groundwater resources in Iran: putting emphasis on Lorestan province as an example in the country. Sustainable Water Resources Management, 9(3), 95
- Mkhinini, M., Boughattas, I., Alphonse, V., Livet, A., Gıustı-Mıller, S., Bannı, M., & Bousserrhıne, N. (2020). Heavy metal accumulation and changes in soil enzymes activities and bacterial functional diversity under long-term treated wastewater irrigation in East Central region of Tunisia (Monastir governorate). Agricultural Water Management, 235, 106150.
- Janeiro, C. A. N., Arsénio, A. M., Brito, R. M. C. L., & van Lier, J. B. (2020). Use of (partially) treated municipal wastewater in irrigated agriculture; potentials and constraints for sub-Saharan Africa. Physics and Chemistry of the Earth, Parts A/B/C, 118-119, 102906
- Daud, M. K., Nafees, M., Ali, S., Rizwan, M., Bajwa, R. A., Shakoor, M. B., . . . Zhu, S. J. (2017). Drinking Water Quality Status and Contamination in Pakistan. BioMed Research International, 2017, 7908183.
- van Steenbergen, F., Kaisarani, A. B., Khan, N. U., & Gohar, M. S. (2015). A case of groundwater depletion in Balochistan, Pakistan: Enter into the void. Journal of Hydrology: Regional Studies, 4, 36-47.
- Jinturkar, A. M., Deshmukh, S. S., Agarkar, S. V., & Chavhan, G. R. (2010). Determination of water quality index by fuzzy logic approach: a case of ground water in an Indian town. Water Science and Technology, 61(8), 1987-1994.
- Braune, E., & Xu, Y. (2010). The Role of Ground Water in Sub-Saharan Africa. Groundwater, 48(2), 229-238.
- Velis, M., Conti, K. I., & Biermann, F. (2017). Groundwater and human development: synergies and trade-offs within the context of the sustainable development goals. Sustainability Science, 12(6), 1007-1017.
- Kumar, P., Kumar, M., Ramanathan, A. L., & Tsujimura, M. (2010). Tracing the factors responsible for arsenic enrichment in groundwater of the middle Gangetic Plain, India: a source identification perspective. Environmental Geochemistry and Health, 32(2), 129-146.
- Dorji, U., Tenzin, U. M., Dorji, P., Wangchuk, U., Tshering, G., Dorji, C., . . . Phuntsho, S. (2019). Wastewater management in urban Bhutan: Assessing the current practices and challenges. Process Safety and Environmental Protection, 132, 82-93.
- Burri, N. M., Weatherl, R., Moeck, C., & Schirmer, M. (2019). A review of threats to groundwater quality in the anthropocene. Science of The Total Environment, 684, 136-154.
- Mishra, R. K. (2023). Fresh Water availability and Its Global challenge. British Journal of Multidisciplinary and Advanced Studies, 4(3), 1-78.
- Bhateria, R., & Jain, D. (2016). Water quality assessment of lake water: a review. Sustainable Water Resources Management, 2(2), 161-173.
- Irfeey, A. M., Najim, M. M. M., Alotaibi, B. A., & Traore, A. (2023). Groundwater Pollution Impact on Food Security. Sustainability, 15(5).
- Boateng, T. K., Opoku, F., & Akoto, O. (2019). Heavy metal contamination assessment of groundwater quality: a case study of Oti landfill site, Kumasi. Applied Water Science, 9(2), 33
- Nyenje, P. M., Foppen, J. W., Kulabako, R., Muwanga, A., & Uhlenbrook, S. (2013). Nutrient pollution in shallow aquifers underlying pit latrines and domestic solid waste dumps in urban slums. Journal of Environmental Management, 122, 15-24.
- Kurwadkar, S. (2017). Groundwater Pollution and Vulnerability Assessment. Water Environment Research, 89(10), 1561-1577.
- Bondu, R., Kloppmann, W., Naumenko-Dèzes, M. O., Humez, P., & Mayer, B. (2021). Potential Impacts of Shale Gas Development on Inorganic Groundwater Chemistry: Implications for Environmental Baseline Assessment in Shallow Aquifers. Environmental Science & Technology, 55(14), 9657-9671.
- Sunitha, V., & Reddy, B. M. (2022). Geochemical characterization, deciphering groundwater quality using pollution index of groundwater (PIG), water quality index (WQI) and geographical information system (GIS) in hard rock aquifer, South India. Applied Water Science, 12(3), 41.
- Shukla, S., & Saxena, A. (2021). Appraisal of Groundwater Quality with Human Health Risk Assessment in Parts of Indo-Gangetic Alluvial Plain, North India. Archives of Environmental Contamination and Toxicology, 80(1), 55-73.
- Wei, M., Wu, J., Li, W., Zhang, Q., Su, F., & Wang, Y. (2022). Groundwater Geochemistry and its Impacts on Groundwater Arsenic Enrichment, Variation, and Health Risks in Yongning County, Yinchuan Plain of Northwest China. Exposure and Health, 14(2), 219-238.
