Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disorder associated with aging and ultimately leads to death. It is marked by a gradual decline in brain function, deterioration of cholinergic activity, and memory loss. AD is among the most prevalent forms of dementia affecting the elderly population worldwide. According to the 2019 World Alzheimer’s Report, nearly 50 million people around the globe were living with dementia, and this number is expected to rise to 152 million by 2050, doubling approximately every 20 years. The symptoms of AD include memory loss, cognitive impairment, behavioral changes, and various psychiatric disorders. Currently, the main pharmacological treatments for AD involve acetylcholinesterase inhibitors and N-methyl-D-aspartate (NMDA) receptor antagonists.
Leucaena leucocephala (Lam.) de Wit, a well-known fodder plant belonging to the Fabaceae (Leguminosae) family, is widely cultivated in Tamil Nadu, India. Besides its agricultural use, this plant possesses notable therapeutic properties, attributed to its diverse phytochemical constituents. Native to southern Mexico and northern Central America, Leucaena leucocephala is a fast-growing tropical tree that has now spread extensively across tropical and subtropical regions. Various parts of the plant—including the leaves, flowers, roots, and bark—demonstrate considerable medicinal potential, exhibiting antifungal, anthelmintic, anticancer, antioxidant, and antidiabetic activities.
The primary objective of the present study was to investigate the potential anti-Alzheimer’s activity of the chemical constituents found in the ethanolic leaf extract of Leucaena leucocephala.
Materials and Methods
Chemicals and Instruments
No purification was done before using any chemicals or solvents of the analytical grade. A soxhelt apparatus was used for the extraction of leaves. Thin layer chromatography (TLC) and column chromatography were used, respectively, to separate and isolate the chemical constituent. IR, 1HNMR, 13CNMR and mass spectroscopy was utilized to characterize the chemical constituents.
Plant Collection and authentication
To prepare the ethanolic extract, fresh, healthy leaves of Leucaena leucocephala were procured from nearby Moradabad districts. The plant material has been submitted as a voucher specimen in the Herbarium of NISCAIR, New Delhi, India. The specimen number associated with the voucher is 3914-15-2.
Extraction
Leucaena leucocephala fresh leaves were air dried for about two weeks at room temperature in the shade. The leaves were powdered once they had thoroughly dried. The extraction process employed a Soxhlet extraction apparatus. Within the apparatus’s upper chamber, a porous thimble held one hundred g of powdered leaf material. To the lower boiling flask, two hundred g of extracting solvent were added. A thermostat-controlled heating mantle was used to heat the flask. The round-bottom flask was filled with ethanol, and the solvent’s boiling point was used to determine the temperature. After heating the solvent to reflux, it was extracted. Up till a dark green extract was gathered on top of the extractor, the material in the thimble was extracted one at a time. Following collection, the ethanolic extract was concentrated independently under low pressure. Following the extraction of ethanol, the extract was weighed and stored at 5 °C in a brown, sealed bottle until needed.
Phytochemical Analysis
The ethanolic extract underwent phytochemical examination to identify various components active secondary metabolites or components like tannins, alkaloids, flavonoids, terpenoids, steroids, carbohydrates, proteins, and saponins11.
Isolation and characterization
TLC experiment was conducted on pre-coated Merck silica gel 60 PF254 aluminium sheets, and column chromatographic separation was executed with Merck 7734 silica gel (60–120 mesh). Iodine vapors helped to visualize the spots. The solvent system consisted of toluene, ethyl acetate, chloroform, and glacial acetic acid (4:3:2:1). The melting point was observed using the open capillary tube method, which is the standard method. Uncorrected results were noted for the findings. Using a Bruker Avance Neo 500 MHz spectrometer in DMSO, the 1HNMR spectra were captured. The internal standard that was employed was Tetramethylsilane (TMS). The 1HNMR chemical shifts were measured from TMS downfield in parts per million (ppm). The internal standard that was employed was Tetramethylsilane (TMS). Utilising a Bruker Avance Neo 500 MHz spectrometer in DMSO, the 13CNMR spectra was captured. The mass spectrum was recorded using a mass spectrometer. Using spectroscopic methods, the structures of the chemical derived from the ethanolic extract were clarified12.
Biological activity
Experimental Animals
Male and female albino rats weighing 140–200 g were used in biological research. The rats were acquired from the IFTM University’s Institutional Animal House in Moradabad, India. Before the trial began, the animals spent seven days in a lab setting. The animals were kept at room temperature of 24±1°C, with a dark and light cycle (12/12 h). The animals were randomly assigned to control and experimental groups during the acclimation period. The animals were kept apart on sterile rice husk bedding in polyacrylic cages. Every animal received an ad libitum supply of fresh water and a normal pellet meal. The Animal Ethical Committee, Committee for the Control and Supervision of Experiments on Animals (CPCSEA), Government of India, examined and approved the protocols for animal-based experiments. The clearance number for these protocols is 2021/837ac/PhD/02.
Acute Toxicity
Using male Wistar albino rats, the acute toxicity of an ethanolic extract was assessed in accordance with Organisation for Economic Co-operation and Development (OECD) guideline number 423. Two groups of six animals each were used for the experimental animals. Before the trial, they fasted for 12 hours while having unrestricted access to water. One group received a single oral dosage of 2000 mg/kg of ethanolic extract, whereas the other received normal saline as treatment. After administering the ethanolic extract, the treated animals were monitored continuously for 30 minutes to check for any changes in overall behaviour, water and food intake, and death. Thereafter, they were monitored sporadically for 4 to 24 hours. The rats were monitored again for a maximum of 14 days after the treatment13.
Biological Evaluation
Nootropic Activity
Nootropic activity evaluation was done by using Y Maze and Novel objective recognition test.
Novel object recognition test (NORT)
Six groups of animals were formed, each group comprising six animals as follows:
Group 1: Normal control Group 2: Scopolamine (Scop) 1mg/kg/day Group 3: Scop + EEAC 100mg/kg/day Group 4:Scop (1mg/kg/day) + EEAC 200mg/kg/day, Group 5:Scop (1mg/kg/day) + EEAC 400mg/kg/day Group 6:Scop (1mg/kg/day) + Donepezil (5mg/kg/day). Treatment was given to rats for 21 days.
Apparatus was a white-coloured open box of plywood. The box was l70 cm in length, 50 cm wide and 40 cm in height. Experiments carried out in three phases: habituation, familiarization, and testing. To habituate and diminish the rats’ dread of a new environment, they were exposed to the device without items for 5 minutes, with the freedom to explore the equipment. The rats were placed in the same setting the next day, but with two identical items A1 and A2 (plastic balls). The familiarization exploring time was 5 minutes. After 24 hours the rats were introduced for testing phase. During this phase, rats were returned to the apparatus, which contained a novel item (Plastic Square) utilized to modify A2. When the rat smelled or made contact with the things with their nose, this was termed exploration. The contact timeplastic square and the plastic ball were noted. To hide any smell residues, the device was washed with disinfectant at the end of each session. The discrimination index/Memory Index (DI) was calculated by using below formula [14].
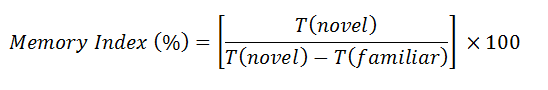
Y Maze
Animals were grouped as per the NORT task. This apparatus employed to estimate percentage alteration (Memory Index) of the experimental rats. Y maze used in this experiment contains three similar arms, P, Q and R. The arm was 40 centimeter in length, 35 cm in height and 12 cm in wirth, positioned at 120ᵒ angles. For the test, every animal was sited on maze triangle center and permitted to freely explore it for 8 minutes. The arm entry was pronounced complete if all the body part completely in the arm. Series of arm entries was visually recorded. To avoid odors, the maze floor was washed with 70% ethanol between trials. The overall number of visits was utilized to calculate the degree of percentage alternation (memory index) using the following formula [15].
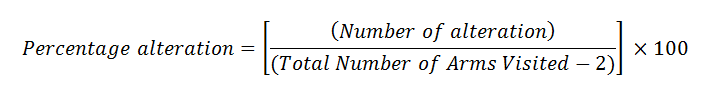
Results and Discussion
Extraction yield (%) and physical characteristics of extracts of leaves
Extraction of leaves was done by using ethanol. It was obtained that the ethanolic extract’s percentage extraction yield was 8.36% w/w. The ethanolic extract showed dark brownish colour. The consistency of ethanolic extract was found to be sticky.
Preliminary phytochemical screening
A standard process was used to check for phytochemicals. Preliminary phytochemical screening of ethanolic extract showed the presence of carbohydrates, alkaloids, saponins, tannins, flavonoids, fats, fixed oils and phenols and presented in table 1.
Table 1: Phytochemical Screening of ethanolic extract of leaves
| Sr. No. | Phytoconstituent Class | Test Name | Results |
| 1 | Carbohydrates | Fehling’s | + |
| 2 | Alkaloids | Dragendroff’s | + |
| 3 | Saponins | Foam Test | + |
| 4 | Test for Steroids | Salkowski Reaction | – |
| 5 | Tannins | Ferric Chloride test | + |
| 6 | Tests for Glycosides | Borntrager’s Test | – |
| 7 | Flavonoids | Shinoda test | + |
| 8 | Test for Fats and Fixed oils | CuSO4 | + |
| 9 | Test for Phenol | Ferric chloride test | + |
Thin Layer chromatography and Column Chromatography
Using the solvents Toluene, Ethyl Acetate, Chloroform, and Glacial Acetic Acid in the proportions 4:3:2:1 (v/v), a TLC finger print profile was established. Using an iodine chamber, five spots were seen (Fig. 1). Compound LLQ was the only one of the five that could be satisfactorily extracted using column chromatography and eluted with n-hexane and chloroform.
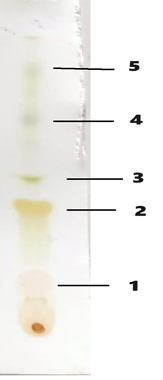
Fig. 1. Pictogram of developed TLC plate of ethanolic extract of Leucaena leucocephala
Characterization
Characterization of isolated compound was done by using IR, 1H NMR, 13C NMR and Mass spectroscopy and spectral data are presented below
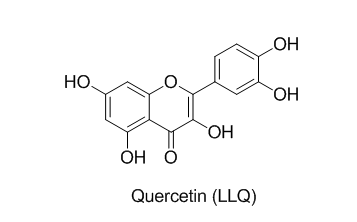
Colour: Yelow, M.P: 314-16 ᵒC, IR (KBr) cm-1: 1517 (C-C stretching),1467 (C=C stretching), 2997 (C-H stretching), 1649 (C=O stretching), 1319 (C-O stretching), 1612 (O-H stretching);1HNMR (500MH2, DMSO-d6): 6.40-7.68- (5H, M, Aromatic); 5.05-5.52- (5H, S, OH); 13CNMR (500MH2, DMSO-d6): 93.45, 98.28, 103.09, 115.15, 115.69, 120.10, 122.07, 135.80, 145.11, 146.86, 147.75, 156.22, 160.78, 163.96, 175.89; MS (FAB) [M + 1]+m/z): 303.02
Compound LLQ was identified as Quercetin by comparison of physical parameters, IR spectra, 1H-NMR spectra, 13C NMR spectra and Mass Spectra with reported data.
Observations for acute oral toxicity studies
During the 14-day observation period in the acute toxicity investigation, oral administration of a single dose up to 2000 mg/kg did not result in any death or toxicity-related symptoms. There were no discernible alterations in the body weight increases. Therefore, Leucaena leucocephala may be safe in doses up to 2000 mg/kg of animal weight. Table 2 displays the parameters that were observed for the acute toxicity research following the administration of the test plant extract in comparison to the normal group.
Table 2: General appearance and behavioral observations of acute toxicity study for control and treated groups.
| Observation | Control Group | Treated Group (2000 mg/kg) |
| Body weight | – | – |
| Body Temperature | – | – |
| Food intake | – | – |
| Urination | – | – |
| Rate of respiration | – | – |
| Change in skin | – | – |
| Eye color | – | – |
| Sedation | – | – |
| Drowsiness | – | – |
| Diarrhea | – | – |
| Coma | – | – |
| Death | – | – |
+ = Present, – = Absent
Nootropic Activity
Novel object recognition test (NORT)
NORT results are exhibits in figure 2. Figure 2 depicts the percentage memory index on the NORT appratus across the treatment groups. In the scop group the % memory index was considerably less than the control group (p<0.05). EELL in a dose 200 mg/kg did not produce significant effect but dose 200 and 400mg/kg produce significant effect in dose dependent manner and Donepezil 5 mg/kg considerably increased % memory index to the Scop group (p < 0.05). These findings showed that EELL in all dose prevented memory deficits produced by Scop in a dose-dependent way.
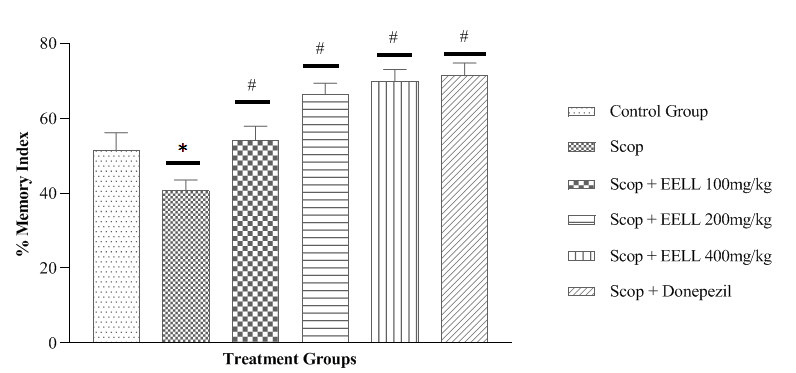
Fig. 2. Effect of EELL on NORT in Scop induced memory impaired rats. Data expressed as mean ± SEM (n=6). *p<0.05 vs control group, #<0.05 vs Scop group.
Y Maze
Y maze results are exhibits in figure 3. Figure 3 depicts the percentage memory index on the Y maze across the treatment groups. In the scop group the % memory index was considerably less than the control group (p<0.05). EELL in a dose 200 mg/kg did not produce significant effect but dose 200 and 400mg/kg produce significant effect in dose dependent manner and Donepezil 5 mg/kg considerably increased % memory index to the Scop group (p < 0.05). These findings showed that EELL in all dose prevented memory deficits produced by Scop in a dose-dependent way.
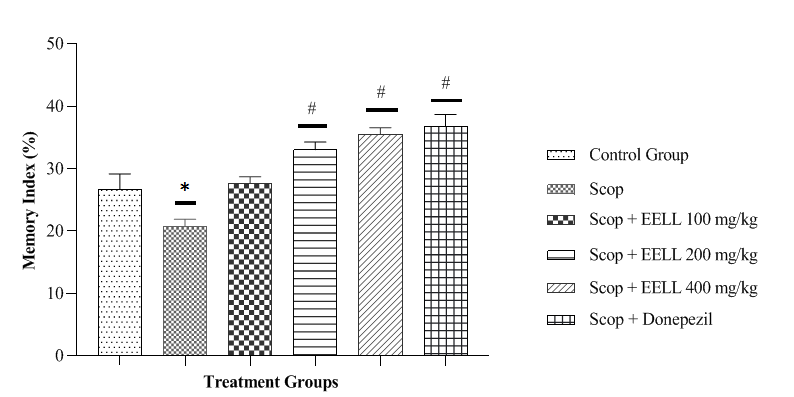
Fig. 3. Effect of EEAC on memory index on Y Maze in Scop induced memory impaired rats. Data represented as mean ± SEM (n=6). *p<0.05 vs control group, #<0.05 vs Scop group.
Conclusion
8.36% w/w percentage yield was obtained from ethanolic extract. A number of phytochemicals such as carbohydrates, alkaloids, saponins, tannins, flavonoids, fats, fixed oils and phenols was found to be present in the ethanolic extract of leave. In acute toxicity study, no toxic effect was found. The results suggested that secondary metabolites were responsible for nootropic activity. The isolation was done from ethanolic extract and spectroscopic methods were used to characterize the isolated compound which demonstrated the chemical structure of compound LLQ as Quercetin.
References
1. Kamel, N.N.; Aly, H.F.; Fouad, G.I.; El-Karim, S.S.A.; Anwar, M.M.; Syam, Y.M.; Elseginy, S.A.; Ahmed, K.A.; Booles, H.F.; Shalaby, M.B.; Maha, Z. Rizk. Anti-Alzheimer activity of new coumarin-based derivatives targeting acetylcholinesterase inhibition. RSC Adv. 2023; 13(27): 18496–18510.
2. Subash, P.; Kareti, S.R.In silico molecular docking analysis for potential anti-Alzheimer’s compounds from the methanolic leaf extract of Erythroxylum monogynum using Gas chromatography–mass spectrometry. J. Saudi Chem. Soc. 2021; 25(8): 101285.
3.Simunkova, M.; Alwasel, S.H.; Alhazza, I.M. Management of oxidative stress and other pathologies in Alzheimer’s disease. Arch. Toxicol 2019; 93: 2491–513.
4.Song, X.; Wang, T.; Guo, L.; Jin, Y.; Wang, J.; Yin, G.; Jiang, K.; Wang, L.; Huang, H.; Long L. In Vitro and In Vivo Anti-AChE and Antioxidative Effects of Schisandra chinensis Extract: A Potential Candidate for Alzheimer’s Disease. Evid. Based Complement Alternat Med. 2020; 2020: 2804849.
5. Deivasigamani, R. Phytochemical analysis of Leucaena leucocephala on various extracts. J. Phytopharmacol. 2018; 7(6): 480-482.
6. Elbanoby, N.; EEl-Settawy, A.A.A.; Mohamed, A.A.; Salem, M.Z.M. Phytochemicals derived from Leucaena leucocephala (Lam.) de Wit (Fabaceae) biomass and their antimicrobial and antioxidant activities: HPLC analysis of extracts. Biomass Conver. Biorefnery. 2022,
7. Widaad, A.; Zulkipli, I.N.; Petalcorin, M.I.R. Anthelmintic Effect of Leucaena leucocephala Extract and Its Active Compound, Mimosine, on Vital Behavioral Activities in Caenorhabditis elegans. Molecules. 2022; 27(6): 1875.
8. Zarin, M.A.; Wan, H.Y.; Isha, A.; Armania, N. Antioxidant, antimicrobial and cytotoxic potential of condensed tannins from Leucaena leucocephala hybrid-Rendang. Food Sci. Human Wellness. 2016; 5(2): 65-75.
9. Benjakul, S.; Kittiphattanabawon, P.; Sumpavapol, P.; Maqsood, S. Antioxidant activities of lead (Leucaena leucocephala) seed as affected by extraction solvent, prior dechlorophyllisation and drying methods. J Food Sci Technol. 2014; 51(11): 3026–3037.
10. Chowtivannakul, P.; Srichaikul, B.; Talubmook, C. Antidiabetic and antioxidant activities of seed extract from Leucaena leucocephala (Lam.) de Wit. Agricul. Natural Resour. Volume 2016; 50(5): 357-361.
11. Kokate CK. Practical Pharmacognosy. 4th ed. New Delhi: Vallabh Prakashan; 1999.
12. Arora, S.; Itankar, P. Extraction, isolation and identification of flavonoid from Chenopodium album aerial parts. J. Tradit. Complement. Med. 2018; 8 (4):476–482.
13. OECD OECD 423 – Guidelines for the testing of chemicals Acute oral toxicity –Fixed dose procedure. Animals. 2001:1–14.
14. Kheradmand, E.; Moghaddam, A.H.; Zare, M. Neuroprotective effect of hesperetin and nano-hesperetin on recognition memory impairment and the elevated oxygen stress in rat model of Alzheimer’s disease. Biomed. Pharmacoth. 2018; 97:1096-1101.
15. Nazir, N.; Zahoor, M.; Nisar, M.; Karim, N.; Latif, A.; Ahmad, S.; Uddin, Z. Evaluation of neuroprotective and anti-amnesic effects of Elaeagnus umbellataThunb. On scopolamine-induced memory impairment in mice. BMC Complement Med. Thera. 2020; 12: 143.
