INTRODUCTION
In the last decade, the heterogeneous catalytic oxidation of hydrocarbons has occupied one of the central places in the modern chemical and petrochemical sciences. This has been prompted by deep research conducted in this area, up to the study of elementary acts and stages of catalysis [1-4].
The study of kinetic regularities and the mechanism of catalytic reactions made it possible to accumulate a lot of experimental material for theoretical generalizations, which contributes to the formation of general concepts and theories of catalysis. Despite the high level of development of oxidative heterogeneous catalysis, some classes of compounds and derivatives of hydrocarbons that could be of interest for catalysis have not yet been the objects of research. One of these objects is the widespread halogen-containing hydrocarbons and, in particular, chlorine-containing hydrocarbons obtained from petroleum hydrocarbon feedstock [5–7].
When carrying out heterogeneous catalytic reactions, one of the main points is the choice of research methods that provide maximum information and accurate analyzes about the rate of stages, the nature of surface components and active centers on the surface of catalysts.
In view of the foregoing, the goal of our research is the catalytic oxidation of chloroaromatic hydrocarbons in order to obtain mono- and dichloromaleic anhydrides (m-ClMA and d-ClMA) for their recovery, as well as clarifying the kinetic regularities of oxidation reaction behavior.
EXPERIMENTAL PART
The study of the kinetics of chemical reactions in an enclosed volume, as well as the static methods, is described in detail in [2-4]. Also, the circulation method used to study the kinetics, which eliminates external diffusion factors, is described within the framework of the static method in detail [5]. Possessing high capabilities, the (integral nature) static method does not provide a precise the activity-change control in catalyst.
The study of the reaction kinetics of flow system makes it possible to carry it out in a stationary mode, which means that, all quantitative characteristics of the system remain unchanged over time at each given point of the reaction space [8–12].
Experiments on study the effect of various variable parameters and kinetic features of the heterogeneous catalytic oxidation of chlorohydrocarbons on various catalytic systems have been carried out on laboratory facilities using reactors with both stationary and fluidized layer of catalysts.
Oxidation of chlorobenzenes and chlorotoluenes has been carried out on the surface of V–P–O/Al2O3, V–Mo–O/ Al2O3, V–Mo–O/ SiO2, V–P–O/SiO2 catalytic systems. 1-monochlorobenzene (1-m-ClB), 1,2-dichlorobenzene (1,2-d-ClB), 1,3,5-trichlorobenzene (1,3,5-t-ClB), 2-monochlorotoluene (2-m-ClT), 2,3-; 2,4- and 2,5-dichlorotoluene (2,3-, 2,4-and 2,5-d-ClT) have been subjected to oxidation [13, 14].
The diagram of the flow laboratory setup for the oxidation of chlorohydrocarbons is shown in Fig.1
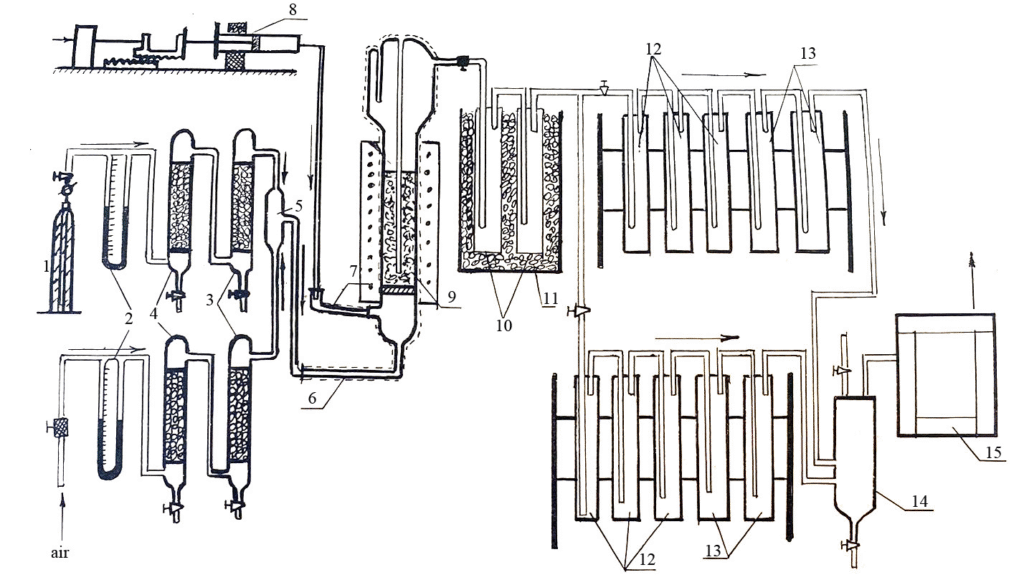
Figure 1. Scheme of an experimental laboratory setup for the oxidation of chlorotoluene. 1 – N2 gas cylinder, 2 – rheometers, 3 – Al2O3 dryers, 4 – CaCl2 dryers, 5 – mixer, 6 – air heater + N2, 7 – evaporator for chlorine toluene, 8 – automatic batcher, 9 – fluidized bed reactor, 10 – trap for products, 11 – ice cooler, 12 – KJ traps for Cl2, 13 – KOH traps for CO2, 14 – CO sampler, 15 – chromatograph.
Air (or O2 and N2) taken for carrying out the oxidation of chloro-hydrocarbons (Cl-HC) at different ratios of chloro-hydrocarbons and oxygen Cl-HC:O2 is heated (6) passing through the suitable dryers (3) and the mixer (5) and supplied to the reactor (9) with a fluidized (or stationary) layer of the catalyst. There, oxidizable chloro-hydrocarbons are supplied using a syringe dispenser (8) and an evaporator (7). The temperature in the reactor is measured using a thermocouple placed in a “pocket”. After the reactor, the reaction gases enter the traps (10) with ice, where the obtained products of the oxidation reaction and the unreacted part of the hydrocarbons are trapped. The non-condensed part of the reaction mixture enters multiple traps (12,13) for chemical analysis of the obtained Cl2 and CO2. The obtained CO and CO2 after absorption of Cl2 by potassium iodide can also be analyzed chromatographically (15). The starting and obtained products were analyzed chromatographically.
The realization of the heterogeneous catalytic reactions using flow and flow-circulation methods carried out in the stationary regime facilitates the kinetic and mathematical description of the regularities of the processes [2, 3]. It is noted that the reaction proceeds on the surface of the solid, where the concentration of the mixture components differs from the concentration inside of catalyst and the gas flow.
The study of kinetic regularity of oxidation reaction of chloro-hydrocarbons is important to establishing the direction and mechanism their oxidation, as well as to build the kinetic models [1–4].
RESULTS AND THEIR DISCUSSION
As previously stated, the study objects were cholorotoluenes (Cl-T) and chlorobenzenes (Cl-B). The investigation of kinetic regularity of these compounds is especially interesting, because the role of the aromatic ring and amount and position of the chlorine atoms in the molecules during their oxidation to obtain monochloromaleic (m-ClMA) and dichloromaleic (d-ClMA) anhydrides are explained. The determination of the condition of the deep oxidation reaction and comparison of the deep oxidation rate with formation reaction rate of the targeted product are also important.
The effect of temperature in the range of 673–773 K, contact time 0.2–1.2 sec, concentration of chlorobenzenes and chlorotoluenes 2,0·103–8,0·10-4mol/l, oxygen concentration 1,0·103–8,0·10-4 mol/l on kinetics of oxidation process has been studied. The kinetic curves of the dependence of the oxidation reaction of chlorotoluene are shown on Figure 2.
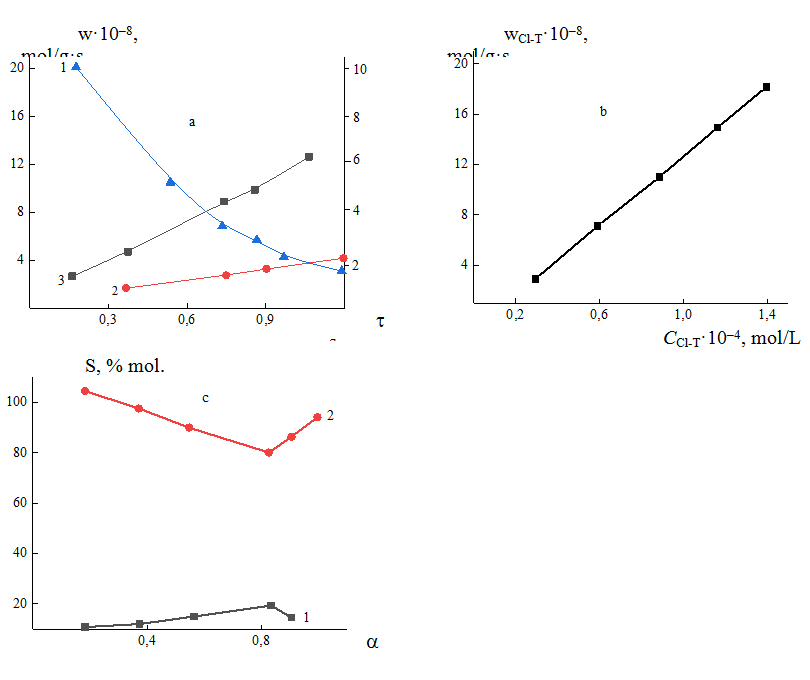
Fig. 2. Kinetic curves of dependence of oxidation reaction rate of Cl-T on the surface of the V–P–O/SiO2 catalyst on contact time (a) 1-Cl-T, 2-m-ClMA, 3-Cl2; on concentration of Cl-T (b); dependence of selectivity on conversion of Cl-T, 1-m-ClMA,2-CO2(c) on the V-P-O/SiO2 surface.
As is seen from figure 2, the oxidation rate of initial chlorotoluene increases gradually, the rates of formation of m-ClMA , release of CO2 and gaseous chlorine on the V-P-O/SiO2 surface increase at the same time with an increase in contact time 0.3-1,2 sec.
The rate of the deep oxidation reaction exceeds the rate of the main reaction of formation of m-ClMA by more than an order of magnitude, however, there is some parallelism in the graphs of these rates up to contact time of τ=0.6 sec, and then increases sharply. The description of the results in coordinates for the corresponding substances shows that they comply with a linear relationship (Fig.2 b). Comparison of the reaction rates of deep and partial oxidation shows that CO2 is formed mainly from the initial chlorotoluene (Cl-T). The ratio decreases to τ=0.8 s, and then begins to increase (Fig. 2), which demonstrates that the reaction of 6 proceeds by the parallel-consecutive mechanism.
The results of studying the kinetic regularities of the oxidation reaction monochlorobenzene (m-ClB) on the surface of the V–P–O/Al2O3 catalyst on the surface of V-P-O/SiO2 catalyst are presented in fig. 3.
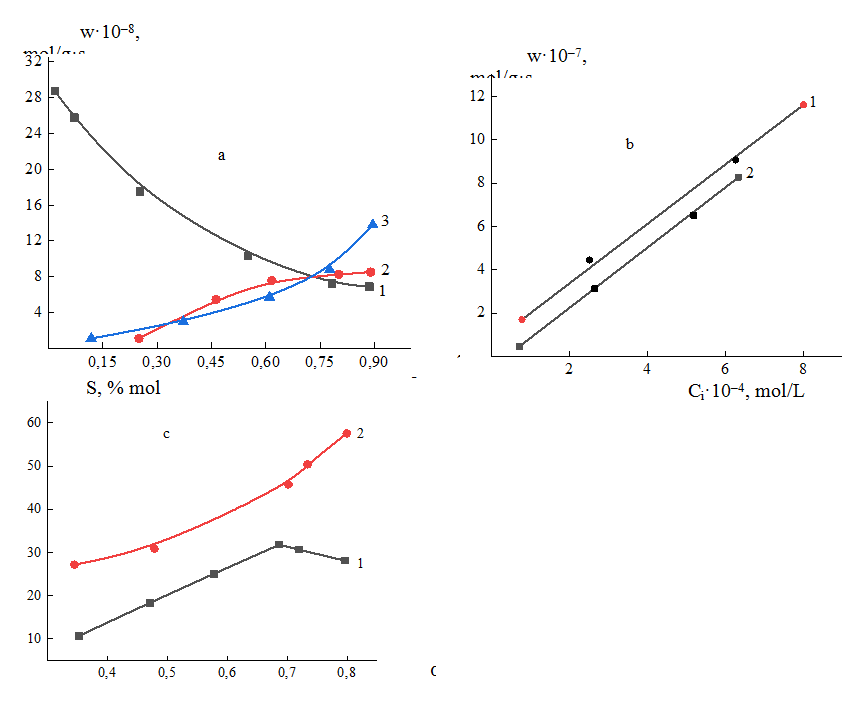
Fig.3. The results of kinetic studies oxidation reaction of m-ClB on the surface of the V–P–O/SiO2 catalyst on the V-P-O/Al2O3 surface, (a) dependence of the rates on contact time 1–m-ClB, 2- m-ClMA, 3-CO2; (b) on concentration of m-ClB; c) dependence of selectivity on conversion of m-ClB, 1- m-ClMA, 2-CO2(c).
As is seen from the presented dependences, the oxidation rate of the initial m-ClB exceeds the rate of d-ClMA formation by an order of magnitude and is comparable to the rate of CO2 formation (a). Carbon dioxide is formed at the beginning of the reaction and further, as well as at small values of contact time τ, but after τ=0.7s, a sharp jump in the rate of CO2 formation is observed. The curve of chlorine formation rate behaves similarly to the curve of CO2 formation rate, which supports the idea that there is an additional pathway for the formation of CO2 from the chlorine-containing compound. This is also seen from Fig. 3(b), where the dependences of the formation rates of CO2 and Cl2 on their concentrations increase in parallel at close values. Comparison of the formation reaction rate of the main d-ClMA and the deep oxidation reaction rate shows that CO2 is formed in a parallel-consecutive scheme both from the initial compounds and from the targeted product under certain conditions, which follows from the analysis of dependence of the selectivity on conversion (Fig. 3c).
The study of the kinetics of oxidation reaction of dichlorobenzenes and trichlorobenzenes on the surface of a vanadium-containing catalyst confirms the above assumption about the formation of CO2. The relationships determined for Cl-T and m-ClB compounds remained same also in this case. Comparison of rates of main reaction and deep oxidation reaction, here, exactly indicates that there is parallel-consecutive pathway of CO2 formation.
The detail investigation of kinetic relationships has been carried out in 1,2-di-chlorobenzene (d-ClB) example to clarify the role of the position of the chlorine atoms in trichlorobenzene (t–ClB). The results of influence of various parameters on the rate of oxidation reaction of dichlorobenzene in stationary, as well as, in fluidized layers of V-P-O/SiO2 catalyst are given in Fig. 4. Comparison of rates of total consumption of dichlorobenzene in stationary and in fluidized layers on the V-P-O/SiO2, V-Mo-O/SiO2 catalysts showed that is 2 times more than under same reaction condition (τ=0.6sec.). The rate of d-ClMA formation is more in this case, whereas the reaction rate of deep oxidation decreases 2 times. Latter goes through minimum in stationary layer, but it is almost stable in fluidized layer (in τ=0.15sec., ; in τ=0.7sec., ) – the increase is slight. According to the obtained results, the oxidation rate of d-ClB in the fluidized layerin pCl-B:O2=1:6 ratio and τ=0.6sec., whereas in d-ClB:O2=1:25. The values of in the fluidized layer (in these ratios) are equal to 7.68 10-8mol/g sec. and 7.44 10-8mol/g sec., respectively. The formation rate of d-ClMA is also more that proves the giving preference to studies of reactions in the fluidized layer. Studies on the oxidation of 1,2-dichlorobenzene, 1,3,5-trichlorobenzene, as well as 3,4-dichlorotoluene and 2,4,6-trichlorotoluene on V–P–O/SiO2, V–P–O/Al2O3, V–Mo–O/SiO2 catalysts showed that in the presence of chlorine atoms in 1,2- or 3,4-positions leads to the formation of DXMA, and in the presence of 1,3,5-position to obtain MXMA, it was also shown that an increase in the number of chlorine atoms increases the rate of formation and selectivity of the targeted acid chlorides. The effect of the concentration of oxygen at various temperatures on the rate of p-ClB oxidation and formation rate of products is presented on Figure 4.
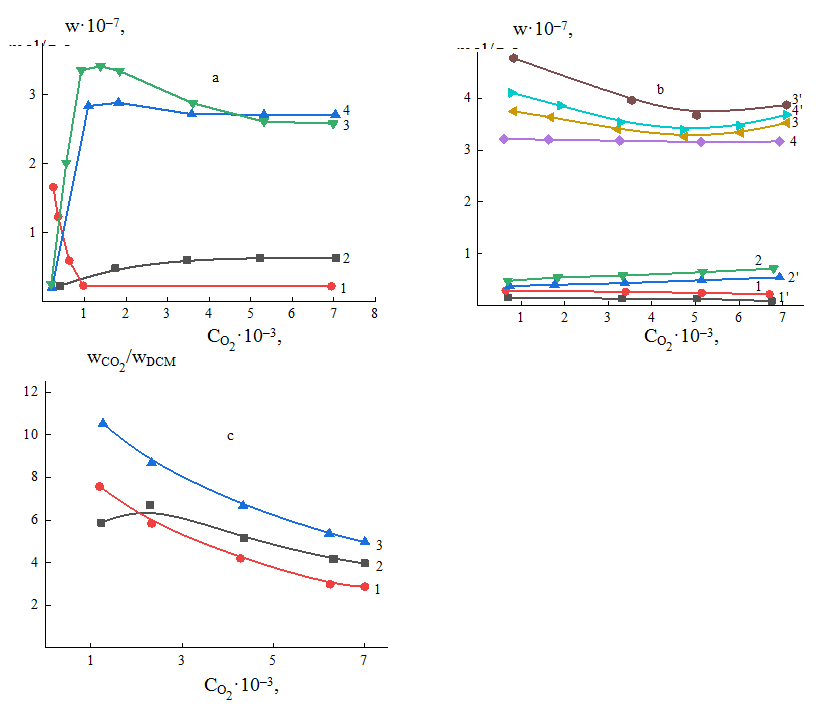
Fig. 4. Theresults of kinetic studies of d-ClB in the fluidized layer of V–Mo–O/SiO2 catalyst at the different temperature, dependence of (a) – on at 713K: 1- , 2- ,3- 4- ; (b) – on at 733K (1,2,3,4) and 733K (1,‘2,‘3,‘4,‘), dependence of on at T,K: 1-713, 2-733, 3-753 (c).
The unusual behavior of dependence of reaction rate on oxygen concentration is observed, especially at very low concentrations of O2 – within 0.08 10-3 mol/L and 0.8 10-3 mol/L, the decrease in the oxidation rate of d-ClB is observed at T=713K.
The further increase in to 7.15 10-3mol/L doesn’t almost affect the oxidation rate of d-ClB. Only the latter slightly increases with the increasing temperature (Figure 4 b). The formation rate of d-ClMA is low at the initial concentration of and increases with raising the up to 5.36 10-3mol/L, finally achieves the maximum. After 713K the increasing temperature slightly goes down. The rate of deep oxidation at initial concentrations of oxygen is low, and increases with rising , achieving the maximum at mol/L, then a little goes down and becomes stable with further increase in . However, the rate of release of free chlorine increases with rising the temperature. Comparison of the reaction rate of d-ClMA formation and the rate of deep oxidation reaction showed that the ratio changes more sharply during the reaction occurring on stationary layer of the catalyst than on fluidized layer (figure 4 c). This ratio increases also with an increase in the temperature [6].
Thus, the study of kinetic regularity of oxidation reaction of di- and trichlorine-containing aromatic hydrocarbons on the surface of vanadium-containing catalysts showed that they have common character. Comparison of the formation rate of products of oxidation and the main (targeted) compounds clearly indicates the presence of two ways of formation – parallel-consecutive. The formation rate of free chlorine and the formation rate of CO2 are likewise, especially after certain values of the contact time and temperature that demonstrates again the ideas about presence of additional way of CO2 formation from both targeted products and obtained chlorine-containing compounds. Comparison of formation rate of targeted products proves that the fluidized layer of the catalyst for realizing the studying reactions is right choice. It has been established that the final products of the reaction don’t have an inhibitory effect on the rate of the oxidation reactions.
Calculations and calculations for this work were performed using the «Оptim Me» software package [15,16].
CONCLUSIONS
The kinetic regularities of the oxidation of chlorine-containing chlorobenzenes and chlorotolyenes on vanadium-containing catalysis of heterogeneous catalytic oxidation on the surface of vanadium oxide catalysts of aromatic chlorohydrocarbons have been studied.
This makes it possible to create a waste-free technology for obtaining valuable polyfunctional compounds, such as mono- and dichloromaleic anhydrous, as well as to utilize the industry waste-products of chlorine aromatic hydrocarbons obtaining during the decomposition process of toxic polychlorohydrocarbons.
It is established that the heterogeneous catalytic oxidation processes of chlorohydrocarbons carried out in the fluidized bed of vanadium-molybdenium-oxide catalysts is way of choice.
REFERENCES
- Kiperman, S.A. Introduction to the kinetics of heterogeneous catalytic reactions. M.: Nauka, 1964. P.514
- Kiperman, S.A., Davidova I.R. Kinetics of para-, ortho-conversion of hydrogen and the application of this reaction to study the mechanism of catalytic reactions. Kinetics and Catalysis. 1961. V.12. №5. P.762-773
- Rozovsky, A.Y. Study of the kinetics of chemical reactions in heterogeneous systems. Diss. doc. chem. sciences. M.: CICI of AS of USSR, p.1966
- Jacques, Védrine. Heterogeneous catalytic partial oxidation of lower alkanes (C 1 –C 6 ) on mixed metal oxides. Journal of Energy Chemistry, Elsevier, 2016. V. 25(6). P.936-946. DOI: 10.1016/j.jechem.2016.10.007
- Slinko, M. M., Makeyev, A. G. Heterogeneous catalysis and nonlinear dynamics. Kinetics and catalysis, 2020. V. 61. № 4. P. 447-468; DOI: 10.31857/S0453881120040140
- Chandler, B. D., Krishnamoorthy, S., Lichtenberger, J. and Amiridis, M. D. On the mechanism of the catalytic oxidation of chlorinated aromatic compounds over supported metal oxide catalysts. Abstr. Pap. Am. Chem. 2000. S. 220, U268-U268.
- Ostrovski, V.E. Oscillation theory of heterogeneous catalysis and its use for identification of the reaction scheme and kinetics: Catalytic liquid-phase benzene-ring hydrogenation as an example. J. Sci. Isr. Technol. Adv. 2012. v.14. p. 57–79
- Ting, Zhang. Heterogeneous Catalytic Process for Wastewater Treatment // Advanced Oxidation Processes . 2020. DOI: 10.5772/intechopen.90393
- Dhakshinamoorthy, A., Alvaro, M. and Garcia, H. Metal-organic frameworks as heterogeneous catalysts for oxidation reactions. Catalysis Science and Technology, 2011. V. 1. No. 6. P. 856–867
- Brazdil, J.F. Selective oxidation in industry: Applications of metal oxides in the petrochemical industry, Chapter 8–2. In Heterogenous Catalysis by Metal Oxides; Védrine, J.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2017.
- Jacques Védrine. Heterogeneous catalytic partial oxidation of lower alkanes (C1 –C 6 ) on mixed metal oxides. Journal of Energy Chemistry, Elsevier, 2016. V.25 (6). P.936-946. 10.1016/j.jechem.2016.10.007. hal-02188605
- Melikova, I.G., Efendiev, A.D., Yunisova, F.A. Reactivity of chlorohydrocarbons in catalytic oxidation reactions. Azerbaijan Chemistry Journal, 2001. No. 2. pp.13–18
- Melikova, I.G., Efendiev, A.J., Yunisova, F.A. Reactivity of chlorohydrocarbons in catalytic oxidation reactions. Azerbaijan Chemistry Journal , 2001. 2,13-18 (in Russian)
- Efendiyev, A.J., Aliyeva, S.A. Development of a process for obtaining dichloromaleic anhydride by direct heterogeneous catalytic oxidation of hexachlorobutadiene. Proceedings of the TPCTI of AS of Az.SSR “Modeling and optimization of chemical processes”, Baku : 1982. P. 4.
- Manafov, M.R. Software application for solving some typical problems of chemical technology. Azerbaijan Chemical Journal. 2016. 2. 89-94
- Manafov, M.R. Development of a Software Application for Solving of Problems of Chemical Kinetics and its Implementation in a C# . Int. J. Eng. Appl. Sci. 2015. 2(10). 33–37.
