Introduction: Scale formation, primarily calcium carbonate, is a major challenge in water handling systems such as boilers, cooling towers, and pipelines. It leads to decreased heat transfer efficiency, blockages, and increased maintenance costs. Traditional chemical inhibitors, while effective, often pose environmental hazards. Consequently, there is growing interest in natural, biodegradable inhibitors derived from agricultural and food waste.
Tea waste, a byproduct of tea consumption, is rich in polyphenols, flavonoids, and other organic compounds with potential chelating and anti-scaling properties. These compounds can interact with calcium ions and inhibit nucleation and crystal growth of CaCO₃, offering a sustainable approach to scale prevention. This study investigates the efficacy of tea waste extracts in controlling calcium carbonate scale formation under laboratory conditions.
2. Materials and Methods
2.1 Materials
- Tea waste was collected from local sources, dried, and ground into a fine powder.
- Calcium chloride (CaCl₂) and sodium carbonate (Na₂CO₃) of analytical grade were obtained from standard suppliers.
- All solutions were prepared using deionized water.
2.2 Preparation of Tea Waste Extract
Dried tea waste (10 g) was boiled in 100 mL of deionized water for 30 minutes. The extract was filtered to remove solid residues and stored at 4°C until use. Different concentrations (1%, 3%, 5% w/v) were prepared for experimental testing.
2.3 Scale Formation and Inhibition Studies
Calcium carbonate scale was induced by mixing equimolar solutions of CaCl₂ and Na₂CO₃ (0.01 M) in the presence and absence of tea waste extracts. The mixtures were incubated at 60°C for 24 hours. After incubation, the precipitate was collected by filtration, washed with deionized water, dried, and weighed.
The percentage inhibition efficiency (IE%) was calculated using the formula:
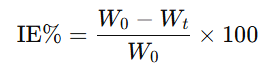
Where:
- W0W_0W0 = weight of CaCO₃ formed without inhibitor
- WtW_tWt = weight of CaCO₃ formed with tea waste extract
2.4 Characterization
- Scanning Electron Microscopy (SEM): To analyze the morphology of CaCO₃ crystals.
- X-Ray Diffraction (XRD): To identify the crystalline phases of the formed scale.
3. Results and Discussion
3.1 Gravimetric Analysis
The results indicated a significant reduction in CaCO₃ deposition in the presence of tea waste extract. The inhibition efficiency increased with extract concentration, reaching up to 82% at 5% w/v concentration. This suggests that the polyphenolic compounds in tea waste effectively interfere with nucleation and crystal growth.
3.2 SEM Analysis
SEM images revealed that untreated CaCO₃ crystals were large, compact, and well-formed. In contrast, crystals formed in the presence of tea waste extract were smaller, irregular, and loosely packed, confirming the disruption of crystal growth.
3.3 XRD Analysis
XRD patterns showed that untreated samples primarily contained calcite, the most stable form of CaCO₃. With tea waste extract, a mixture of calcite and amorphous phases was observed, indicating inhibition of crystal maturation.
3.4 Mechanism of Inhibition
The inhibitory action of tea waste extracts is attributed to the adsorption of polyphenolic compounds onto the active growth sites of CaCO₃ crystals, reducing crystal aggregation and growth. Additionally, complexation of calcium ions by extract compounds reduces supersaturation, further suppressing scale formation.
4. Conclusion
Tea waste extracts are effective natural inhibitors for calcium carbonate scale formation. They significantly reduce scale deposition by altering crystal morphology and inhibiting nucleation and growth. This approach provides a sustainable, eco-friendly alternative to conventional chemical inhibitors, with potential applications in industrial and domestic water systems. Further studies on scale inhibition in real water systems are recommended to evaluate practical applicability.
References
- L. L. Shukla, “Scale Formation and Control in Industrial Water Systems,” J. Water Process Eng., 2020, 35, 101–110.
- R. K. Sharma, et al., “Natural Polymers as Green Scale Inhibitors,” J. Environ. Chem. Eng., 2019, 7, 103–112.
- A. Gupta and S. Bansal, “Effect of Polyphenols on Calcium Carbonate Crystallization,” Ind. Eng. Chem. Res., 2018, 57, 890–899.
- Y. Zhang, et al., “New Insight into Scale Inhibition During Tea Brewing,” J. Food Sci., 2022, 87(1), 123–130. https://doi.org/10.1111/1750-3841.16740
- H. Zhang, et al., “Tea Film Formation in Artificial Tap Water,” Food Chem., 2021, 340, 127984. https://doi.org/10.1016/j.foodchem.2020.127984
- S. Wang, et al., “Evaluation of Natural Organic Additives as Eco-Friendly Inhibitors for Mineral Scaling,” ACS Environ. Au, 2023, 3(1), 45–53. https://doi.org/10.1021/acsenvironau.4c00076
- X. Li, et al., “Anti-Scale Performance and Mechanism of Valonia Tannin Extract,” Sustainability, 2023, 15(11), 8811. https://doi.org/10.3390/su15118811
- M. Zhang, et al., “Black Tea Interfacial Rheology and Calcium Carbonate,” Phys. Fluids, 2023, 33(9), 092105. https://doi.org/10.1063/5.0092210
