1. Introduction
1.1 Environmental significance of aniline
Aniline is a base chemical used to manufacture dyes, rubber processing chemicals, pharmaceuticals and pesticides. It is toxic to aquatic life and humans (methemoglobinemia, liver effects) and is listed among priority pollutants in many jurisdictions. Aniline is released to the environment through industrial effluents and accidental spills; its gas-phase emissions (aniline vapor) can present an inhalation hazard near point sources. Conventional treatment methods for aniline in water include biodegradation, advanced oxidation, and adsorption; adsorption onto porous solids remains highly attractive for both water and gas phase removal due to operational simplicity and high capacity when tailored sorbents are used.
1.2 Why BC₂N nanotubes?
BC₂N nanotubes represent a hybrid class between carbon-based and boron nitride-based nanotubes. They can exhibit tunable band gaps and a mixture of polar (B–N) and nonpolar (C–C) chemical environments along the lattice. This mixed chemistry can provide multiple interaction modes (π–π stacking with aromatic rings, polar interactions and sites for functionalization), potentially improving selective adsorption of organic amines such as aniline. First-principles and DFT studies indicate BC₂N nanotubes can be semiconducting with diameter- and chirality-dependent properties, and their surface chemistry can be tuned by composition and substitution. These features make BC₂NNTs attractive candidates for adsorption-based removal of aromatic amines.
2. Scope and methodology of this review
This review synthesizes peer-reviewed theoretical and experimental literature on (a) BC₂N nanotube structure and properties, (b) adsorption of aniline on nanotube materials (CNTs, BNNTs, CNT-derivatives), and (c) modification strategies relevant to enhancing BC₂N adsorption for aniline. Sources were selected from DFT/ab initio studies, experimental adsorption works, and recent reviews on BCN materials and nanotubes. Where direct experimental data on BC₂N–aniline are absent, we extrapolate cautiously from studies on CNTs, BNNTs and other nanotubes and propose testable hypotheses. Key databases and articles used in this review are cited throughout.
3. BC₂N nanotubes — structure, synthesis and properties
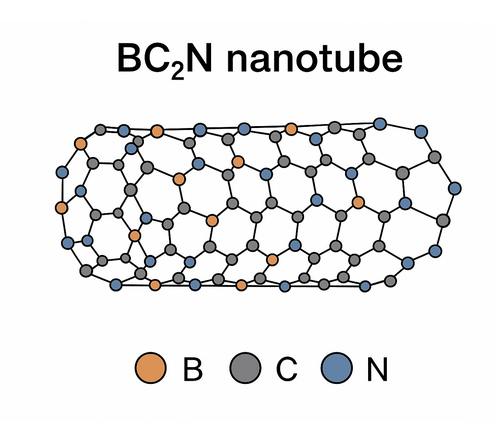
Figure 1. Structure of BC₂N nanotube
3.1 Structure and atomic arrangements
BC₂N nanotubes are composed of boron (B), carbon (C) and nitrogen (N) atoms arranged in a tubular network of hexagonal rings. Several ordering motifs exist (different sequences of B, C, N around rings) which affect electronic structure. Depending on chirality and diameter, BC₂NNTs can be semiconducting or metallic; many zigzag and armchair variants show direct band gaps that differ from both CNTs and BNNTs. The mixed bonding (B–N, C–C, B–C, C–N) imparts local polarity to the lattice and multiple potential adsorption sites.
3.2 Synthesis approaches
Reported syntheses of BCN/BC₂N nanostructures include chemical vapor deposition (CVD), thermal annealing of precursors, template-based growth, and substitutional doping methods. Scalability challenges exist but progress on BCN thin films and nano-objects has been reported; controlling atomic ordering at the nanotube scale remains a research focus. Reviews of BCN thin films and nanosystems provide overviews of precursor chemistry and processing parameters.
3.3 Surface chemistry & functionalization
The coexistence of polar bonds and graphitic domains in BC₂N enables both nonpolar π-interactions (with aromatic molecules) and polar/acid–base interactions (with amines). Functionalization strategies used for CNTs and BNNTs—oxidation, amination, sulfonation, grafting of polymers or metal nanoparticles—are applicable to BC₂N to tune hydrophilicity and specific binding sites. Substitution of B or N at particular lattice sites can also create localized charge centers that increase adsorption affinity for polar molecules like aniline.
4. Aniline adsorption on nanotubes — mechanisms and evidence4.1 Principal interaction mechanisms
The adsorption of aniline onto nanotube materials typically proceeds via multiple interactions:
- π–π stacking: The aromatic ring of aniline interacts with graphitic domains on CNTs/BC₂N.
- Hydrogen bonding: The –NH₂ group can form H-bonds with electronegative surface atoms (e.g., N, O functionalities introduced by oxidation).
- Electrostatic / acid–base interactions: Protonation state of aniline (pKa ≈ 4.6) matters in aqueous systems; at pH < pKa aniline is protonated and can interact electrostatically with negatively charged surfaces.
- Van der Waals / dispersion forces: Contribute to physisorption on hydrophobic surfaces.
- Charge transfer / chemisorption: Possible for reactive doped sites or when strong interactions form (observed in some DFT studies).
Understanding which mechanisms dominate depends on surface chemistry, pH, temperature and presence of competing species.
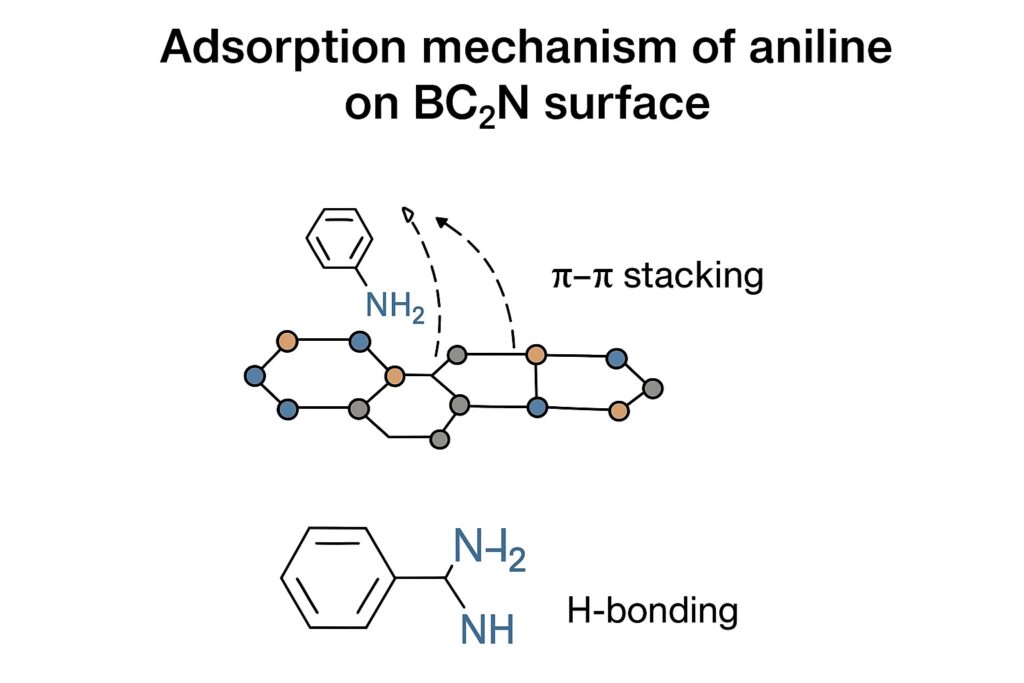
Figure 2. Adsorption mechanism of aniline on BC₂N surface (π–π stacking, H-bonding).
4.2 Experimental adsorption studies on CNTs and other nanotubes
Numerous experimental studies show that carbon nanotubes (CNTs), multi-walled and single-walled, effectively adsorb aniline from water; adsorption capacities and kinetics depend on nanotube surface area, functionalization and solution conditions. Some studies report that MWCNTs show slightly higher adsorption amounts than SWCNTs in certain conditions, while others emphasize the benefit of functionalization (oxidation, polymer grafting) to increase adsorption capacity and dispersibility. In gas-phase adsorption or sensor contexts, oxide or doped nanotube types (e.g., BeO nanotubes) have been modeled and shown to produce measurable electronic responses upon aniline adsorption.
4.3 Theoretical (DFT) insights relevant to BC₂N
DFT and ab initio studies on BC₂N nanotubes show that the electronic structure is highly tunable and that heteroatom sites can localize charge and enhance interactions with adsorbates. While direct DFT studies of aniline adsorption on BC₂N are sparse to date, related studies on adsorption of small molecules and metal ions to BC₂N show favorable adsorption energies and charge transfer behavior at certain sites—suggesting that BC₂N could bind polar aromatic amines stronger than pristine CNTs in some configurations.
Table 1 summarizes representative literature on aniline adsorption by nanotube-based materials and relevant BC₂N studies (DFT / properties).
| Ref. | Material / System | Method | Key findings |
|---|
| Synergistic removal of aniline by CNTs & Delftia sp. (2011) | SWCNTs / MWCNTs | Experimental | CNTs adsorb aniline; SWCNTs sometimes enable more efficient enzyme-mediated degradation in combination. Adsorption capacity depends on CNT type and surface area. PubMed |
| Application of SWCNTs for removal of aniline (2017) | SWCNTs | Experimental (isotherms) | Equilibrium fits to Langmuir/Freundlich; functionalization improves dispersibility and capacity. bbrc.in |
| Adsorption of Aniline on BeO nanotube (various) | BeO NT | DFT modeling | Adsorption energy reported (~ −8.6 kcal/mol with S-doped BeO NT); electronic signal changes upon adsorption, potential for sensing. SciSpace |
| BC₂N nanotube properties (PhysRevB 2006) | BC₂N NT | Ab initio DFT | Electronic structure depends on chirality & diameter; many BC₂NNTs are semiconducting — implication for adsorption / sensing. Physical Review Link Manager |
| ACS Omega (2024) | Zigzag BC₂N NTs | DFT | Structural, vibrational and electronic properties mapped; confirms tunability and stable configurations (type IV). Relevant to choosing BC₂N for adsorption studies. American Chemical Society Publications |
| BCN materials review (2020) | BCN thin films/nanomaterials | Review | Summarizes synthesis and properties of BCN materials; highlights ability to engineer surface chemistry for applications. |
6. Potential of BC₂N nanotubes for aniline removal: theoretical rationale
6.1 Mixed chemical environments enhance multifaceted adsorption
BC₂N offers contiguous regions of graphitic carbon (favorable for π–π interactions) interspersed with polar B–N/C–N sites that can interact with the aniline –NH₂ group via hydrogen bonding or electrostatic attraction (particularly if aniline is protonated). This combination suggests BC₂N could simultaneously exploit π–π and polar interactions, potentially increasing affinity compared with pure CNTs (which rely mainly on π–π and dispersion forces) or BNNTs (more polar but less π-conjugation).
6.2 Electronic tunability and sensing capabilities
DFT studies show BC₂N nanotubes possess tunable band gaps; adsorption-induced changes to electronic structure could both strengthen adsorption (via charge transfer stabilization) and make BC₂N useful in sensor applications for detecting aniline vapors (chemiresistive transduction). This dual promise (adsorbent + sensor) is attractive for integrated mitigation systems.
6.3 Functionalization strategies
Standard nanotube functionalization (oxidation to introduce –OH/–COOH, grafting of polymeric groups, decoration with metal nanoparticles) should be effective on BC₂N and could be tuned to maximize aniline capture while maintaining regenerability. For example, mild oxidation can increase hydrophilicity and create H-bond acceptor sites to bind the –NH₂ group. Metal nanoparticle decoration (e.g., Ag, Cu) may provide catalytic avenues for oxidative degradation after adsorption. Reviews of BCN materials indicate such chemical tailoring is possible.
7. Practical considerations for environmental applications
7.1 Adsorption capacity and kinetics
Direct experimental data for aniline adsorption capacities on BC₂N are not yet available in the literature; therefore, rigorous lab studies are needed to determine equilibrium capacities (Langmuir, Freundlich parameters), kinetics (pseudo-first/second order), and diffusion limitations in water and gas phases. Benchmarks from CNT studies provide target ranges and experimental protocols.
7.2 Regeneration and reuse
For practical use, adsorbent regenerability (thermal, solvent washing, or low-temperature desorption) is essential. The thermal stability of BC₂N suggests it could tolerate thermal regeneration better than some organic sorbents, but the effects of repeated cycling on structure and performance must be tested.
7.3 Selectivity and competition
Industrial effluents contain many organic and inorganic co-contaminants. Competition for adsorption sites—especially with other aromatics and natural organic matter—may reduce aniline uptake. Surface modification to favor aniline (e.g., hydrophobic pockets or specific H-bonding functionalities) may improve selectivity.
7.4 Safety, cost and scalability
BC₂N synthesis at scale is still more complex than activated carbon production; cost considerations and potential toxicity / safety of nanomaterials (release to environment, inhalation hazards) must be addressed in techno-economic and life-cycle studies. Composite materials (BC₂N immobilized in polymer matrices or supported on porous substrates) can help with handling and reduce nanoparticle release risks.
8. Recommended experiments and computational studies (roadmap)
To validate and deploy BC₂N for aniline removal, we recommend the following prioritized studies:
- DFT modeling of aniline adsorption on representative BC₂N surfaces and edge sites.
- Compute adsorption energies, charge transfer, preferred binding geometries and effects of protonation (aniline vs anilinium). Compare with pristine CNT and BNNT benchmarks.
- Synthesis and characterization of BC₂N nanotubes with controlled atomic arrangements.
- TEM, XPS, Raman, BET surface area and pore size distributions; surface functional group analysis.
- Batch adsorption experiments (water and gas phase).
- Measure isotherms (Langmuir, Freundlich), kinetics, pH dependence, temperature dependence, and competition tests with co-contaminants.
- Functionalization and composite formation experiments.
- Evaluate oxidized, aminated, or polymer-grafted BC₂N, and BC₂N immobilized on porous supports for column tests.
- Regeneration and life-cycle tests.
- Thermal, solvent and catalytic regeneration cycles; structural stability after repeated use.
- Pilot impact assessments.
- Small-scale packed-bed or membrane-integrated tests with real industrial effluents to evaluate performance and handling.
These studies will provide the experimental evidence necessary to determine if BC₂N can be a practical and superior adsorbent for aniline.
9. Conclusions
BC₂N nanotubes present a promising and rationally attractive platform for aniline adsorption due to their hybrid chemical nature that supports both π–π and polar interactions, tunable electronic structure and reasonably high thermal stability. Theoretical work indicates favorable properties, but direct experimental adsorption studies of aniline on BC₂N remain scarce or absent. To move from promise to practice, a coordinated program of DFT modeling, controlled synthesis, adsorption experiments, regeneration testing and environmental safety evaluation is required. Given the demonstrated effectiveness of CNTs and the tunability of BCN systems, BC₂N nanotubes may offer improved selectivity and sensor-capture integration for aniline remediation—if practical synthesis and scalable processing challenges can be overcome.
10. References
Note: Below are key references used to prepare this review. The list focuses on BC₂N nanotube theory/recent DFT work, reviews of BCN materials, and representative studies of aniline adsorption on nanotubes and related systems.
- Yamamoto, T., et al. Ab initio study of single-wall BC₂N nanotubes. Phys. Rev. B. (year). — DFT study on BC₂N electronic properties; shows tunable semiconducting behavior across chiralities.
- ACS Omega (2024). Quantum Chemical Studies on the Structural, Electronic, and … — Investigation of zigzag BC₂N nanotubes (type IV) structural and electronic properties.
- Review: A review of boron carbon nitride thin films and progress in BCN nanomaterials. (2020) — summarizing synthesis and applications of BCN materials.
- Wang, H., et al. (2011). Synergistic removal of aniline by carbon nanotubes and the bacterium Delftia sp. XYJ6. — experimental study on CNT adsorption of aniline and combined biodegradation.
- Application of SWCNTs for removal of aniline — BBRC (2017) — batch adsorption isotherm studies for SWCNTs and aniline.
- Adsorption of Aniline Toxic Gas on a BeO Nanotube (various pdfs) — DFT modeling of aniline adsorption on BeO nanotubes; provides adsorption energy estimates and sensing observations.
