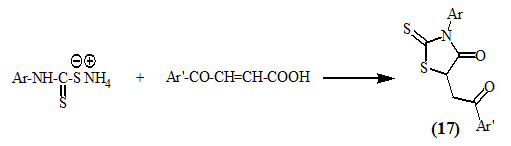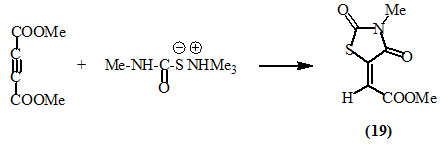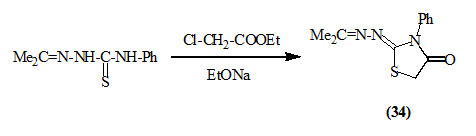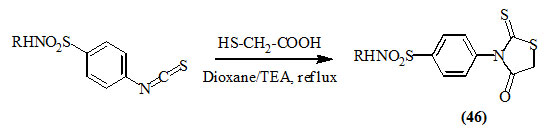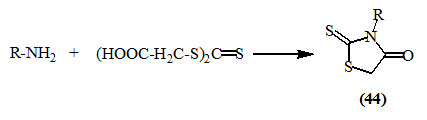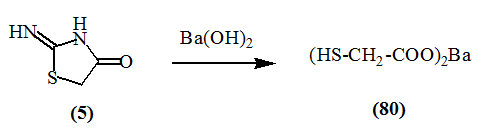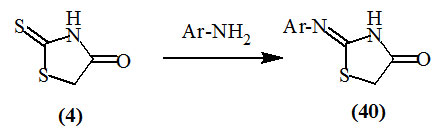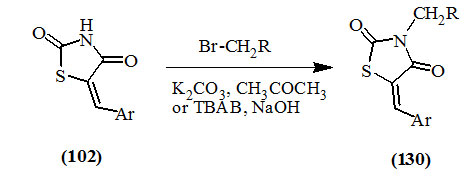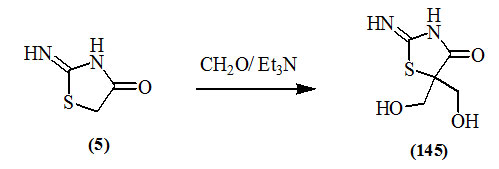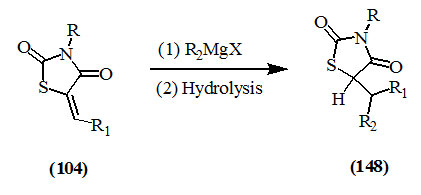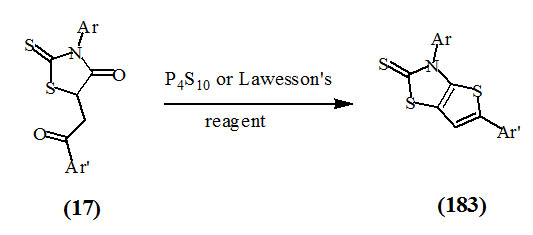Introduction
4-Oxothiazolidine (2) is a derivative of thiazolidine (1) with a carbonyl group at position-4.
4-Oxothiazolidines are an important group of heterocyclic compounds that are used in the field of medicinal chemistry. The utility of 4-oxo-thiazolidines as synthons for various biological compounds has given impetus to these studies. In recent years, 4-oxo-thiazolidines have been among the most extensively studied compounds. 4-Oxothiazolidines have been shown to exhibit various important biological activities such as antibacterial, antifungal, antiviral, diuretic, anti-tubercular, anti-HIV, antihistaminic, anticancer, anticonvulsant, anti-inflammatory, and analgesic properties (Ramadan and El-Helw, 2018; Ramadan and Sallam, 2018; Ramadan and Abou-Elmagd, 2018; Ramadan et al., 2017;2016;2015; Capan et al., 1999). For this reason, a variety of 4-oxothiazolidine compounds have been prepared in this study. Diurno, (1999) prepared a series of 4-oxothiazolidine derivatives and studied their inhibition ability to the specific quantity applied on pig. His study found the new horizon of antihistamine because the synthesized compounds showed excellent results. Emmet et al., (1982) reported twenty derivatives of 4-thiazolidinones as free bases for bio-assays. These derivatives were employed on guinea pig ileum and evaluated for their capability to inhibit the contractions by induced- histamine. They have highlighted thiazolidinone as an essential moiety for antihistaminic drugs.
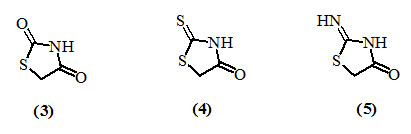
The substituents in the 4-oxo-thiazolidine ring in the 2-, 3- and 5-positions may be varied to give numerous derivatives, but the greatest difference in the properties is exerted by the group attached to the carbon atom in the position-2, to give 2,4-dioxothiazolidine (3), 2-thioxo-4-oxothiazolidine (or rhodanine) (4), and 2-imino-4-oxo-thiazolidine 5, ring systems. Numerous methods for the synthesis of 4-oxo-thiazolidines and also their diverse reactions offer enormous scope in the field of medicinal chemistry.
Synthesis of 4-Oxothiazolidines
From Dithiocarbamates
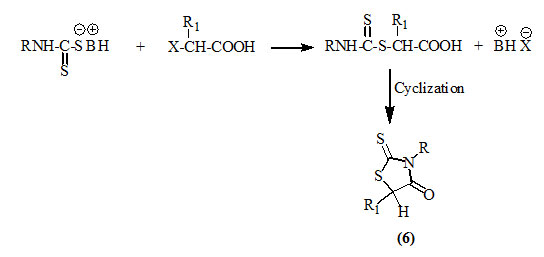
It was reported that dithiocarbamates, obtained from the reaction of ammonia, primary amines (Redemann et al., 1955; Abrahamson et al., 1967; Turkevich and Petlichnaya, 1971; Sahoo et al., 1989 and Toubal et al., 2012) or amino acids (Kashkaval, 1966; 1967) with carbon disulfide in the presence of a base, reacted with α–haloalkanoic acids to afford the respective 2-thioxo-4-oxothiazolidines (6).
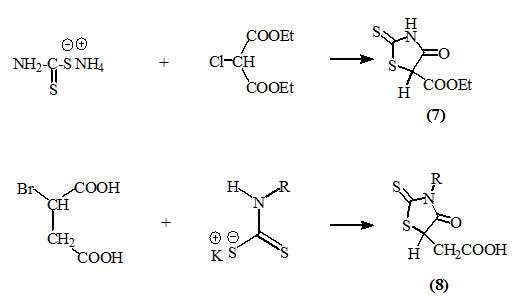
Similarly, α-halodicarboxylic acids or esters such as α-bromosuccinic acid and diethyl chloromalonate were reacted with dithiocarbamates to afford 5-ethoxycarbonyl- (7) and 5-carboxymethyl- (8) 2-thioxo-4-oxothiazolidine derivatives, respectively (Minka, 1964).
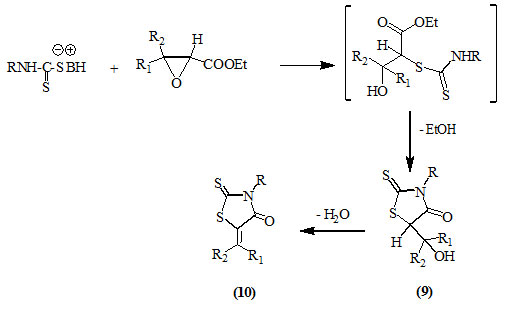
Glycidic esters reacted with dithiocarbamates to afford 3-substituted-5-hydroxyalkyl-2-thioxo-4-oxo-thiazolidines (9) which are readily dehydrated in refluxing acetic acid to produce the respective 5-alkylmethylene derivatives (10) (Roggero and Audibert, 1971).
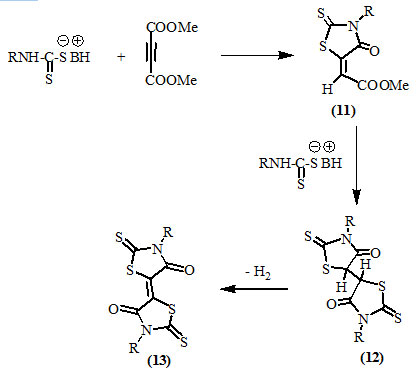
Dimethyl acetylenedicarboxylate (DMAD) reacted readily with dithiocarbamates in methanol under variable conditions to give either 3,3′-disubstituted-2,2′-dithioxo-4,4′-dioxo-∆5,5′-bi-thiazolidines (13) or 5-(methoxycarbonyl-methylene)-2-thioxo-4-oxo-thiazolidines (11). The course of reaction was suggested to involve formation of compounds (11) at first, which reacted with dithiocarbamates to give compounds (12). Autoxidation of (12) afforded (13) (Nagase, 1973).
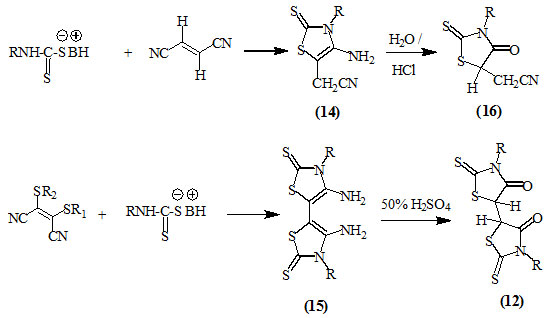
Nagase, (1974) studied the addition reaction to the activated double bonds and reported the formation of compounds (14) and (15) upon the reaction of fumaronitrile and bis(alkylthio)-maleonitrile respectively, with dithio-carbamates. Acid treatment of compounds (14) and (15) resulted into the formation of 4-oxo-thiazolidines (16) and (12) respectively.
Omar et al., (1995; 2000) reported that the reaction of dithiocarbamates with β-aroylacrylic acids furnished 3-aryl-5-(2-aryl-2-oxo-ethyl)-2-thioxo-4-oxo-thiazolidines (17).
From Thiocarbamates
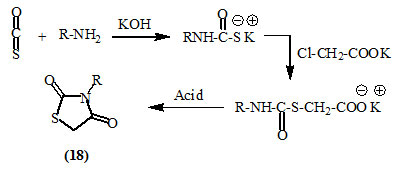
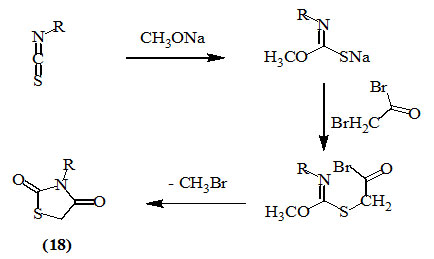
Holmberg, (1909) and Kallenberg, (1923) used thiocarbamates, obtained from carbon oxysulfide and amine, to obtain 2,4-dioxo-thiazolidines (18).
Dimethyl acetylenedicarboxylate (DMAD) reacted with methyl ammonium N-methyl-thiocarbamate in methanol at room temperature to give 3-methyl-5-methoxycarbonyl-methylene-2,4-dioxo-thiazolidine (19) (Nagase, 1973).
From Thiourea Derivatives
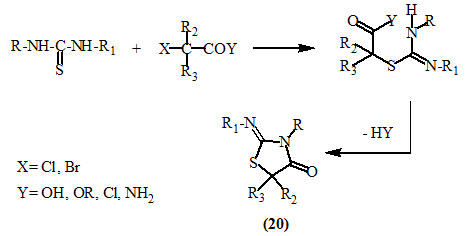
The substituted and unsubstituted 2-imino-4-oxo-thiazolidines (20) were obtained in good yields by the reaction of symmetrical and unsymmetrical thioureas with various α-haloalkanoic acids, esters, acid chlorides, amides, or carbamates (Gupta et al., 1978; Chande and Ambhaikar, 1996; Chande and Bhat, 2000; Sedlàk et al., 2002, 2003; Hanusek et al., 2004 and Prakash et al., 2010).
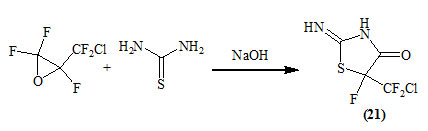
Kvicala et al., (2001) reported the conversion of 2-(chlorodifluoromethyl)-2,3,3-trifluorooxirane into 2-imino-5-(chlorodifluoromethyl)-5-fluoro-4-oxo-thiazolidine (21) by treating with thiourea in the presence of sodium hydroxide.
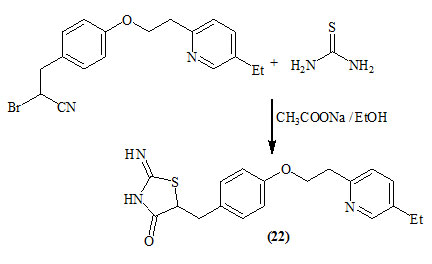
Halama et al., (2002) reported that the reaction of thiourea with 2-bromo-3-[4-[2-(5-ethyl-2-pyridyl)ethoxy]-phenyl]propionitrile in the presence of sodium acetate and ethanol gave 5-[4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl]-2-imino-4-oxo-thiazolidine (22).
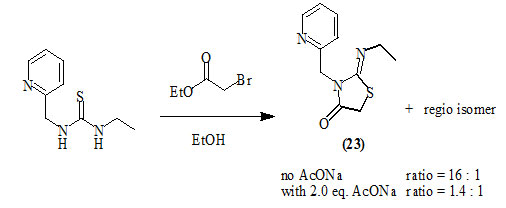
Laurent et al., (2004) reported that the cyclization of unsymmetrical thiourea derivatives with ethyl bromoacetate afforded 3-heteroaryl-2-imino-4-oxo-thiazolidines (23) with excellent levels of regio-control. In the absence of base, 2-(pyridylmethyl) substituent on the thiourea scavenge acid that is generated upon sulfur alkylation with bromoesters, while a completely non-selective cyclization occurred when 2.0 equiv. of sodium acetate (AcONa) was present during the reaction. The resulted conjugate acid played an important role in influencing the regio-chemical outcome and overall rate of the reaction.
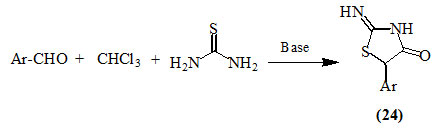
Blanchet and Zhu, (2004) reported that an efficient synthesis of 2-imino-4-oxo-thiazolidines (24) could be achieved from the one-pot three-component reaction of aldehydes, chloroform and thiourea in the presence of a base.
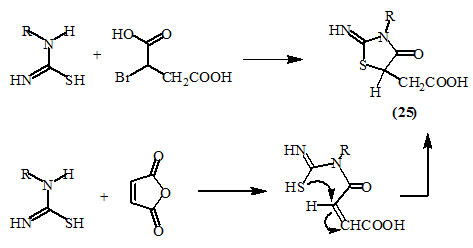
α-Halodicarboxylic acids, such as α-bromosuccinic acid and maleic anhydride yielded 5-carboxymethyl-2-imino-4-oxo-thiazolidines (25) upon the treatment with thiourea derivatives in the presence of sodium acetate and methanol (Deghenghi and Daneault, 1960; Minka, 1966; Trivedi et al., 1966; Raman et al., 1978).
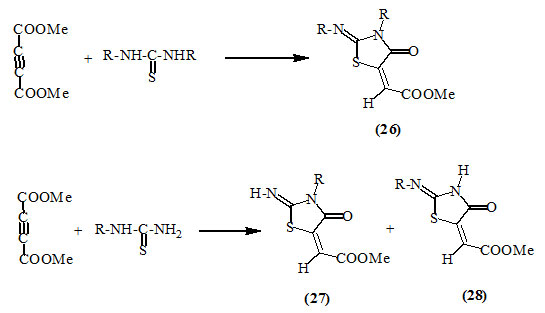
Dimethyl acetylenedicarboxylate (DMAD) reacted readily with the symmetrically substituted thiourea to produce 2-substitutedimino-5-methoxycarbonylmethylene-4-oxo-thiazolidine (26). Monosubstituted thiourea, however, gave isomeric mixtures of the respective 2-imino-4-oxo-thiazolidines (27) and (28) (Lown and Ma, 1967; Nagase, 1973).
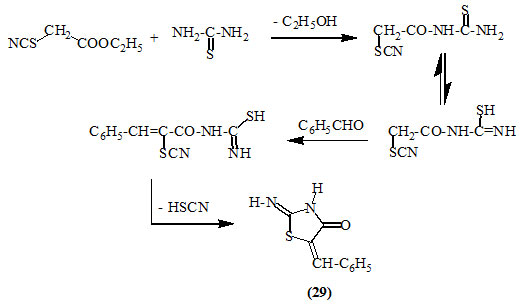
It was reported that thiourea reacted with ethyl thiocyanoacetate and benzaldehyde to afford 2-imino-5-phenylmethylene-4-oxo-thiazolidine (29) (Kambe and Hayashi, 1972).
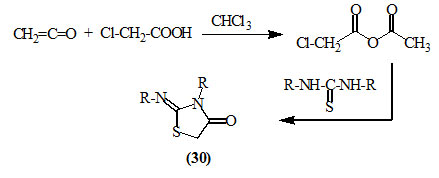
Svetkin et al., (1968) reported that the mixed anhydride, prepared by passing ketene into chloroacetic acid, was reacted with thiourea derivatives in chloroform at 60oC to give various 2-imino-4-oxo-thiazolidines (30).
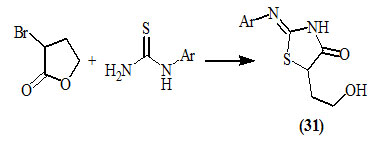
Vàna et al., (2009) synthesized 5-(2-hydroxyethyl)-2-arylimino-4-oxo-thiazolidines (31) by treating arylthiourea with 3-bromotetrahydrofuran-2-one under mild conditions.
From Thiosemicarbazones
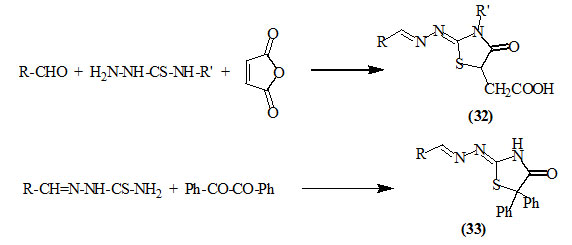
Thiosemicarbazones condensed with maleic anhydride (Mclean and Wilson, 1939; Polovkovych et al., 2010) and/or benzil (Mclean and Wilson, 1939; Saiz et al., 2009) to yield the respective 5-carboxymethyl-2-hydrazino-4-oxo-thiazolidines (32) and 2-hydrazino-5,5-diphenyl-4-oxo-thiazolidines (33). Saiz et al., (2009) developed a tandem method for the synthesis of (32) from commercially available materials in a one pot 3-components reaction. The reaction between aldehydes, thiosemicarbazides and maleic anhydride was effectively assisted by microwave irradiation.
Stephen and Wilson, (1926) reported that the reaction of acetone-4-phenyl-thiosemicarbazone with ethyl chloro-acetate in the presence of sodium ethoxide gave 2-hydrazino-3-phenyl-4-oxo-thiazolidine (34).
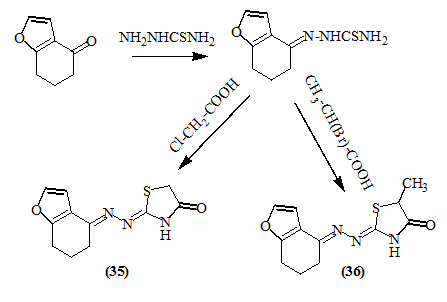
Gautam et al., (2012) reported a convenient solvent-less cyclocondensation from thiosemicarbazone of 6,7-dihydrobenzo[b]furan-4(5H)-one and chloroacetic acid or 2-bromopropionic acid using an ionic liquid, N-methyl-pyridinium tosylate for obtaining 4-oxo-thiazolidines (35) and (36) in excellent yields.
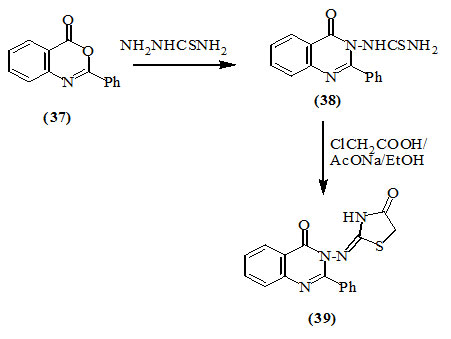
Ameta et al., (2006) synthesized 3-[4-oxo-thiazolidin-2-yliden)amino]-2-phenyl-quinazolin-4(3H)-one (39) via treating 2-phenyl-3,1-benzoxazin-4(3H)-one (37) with thiosemicarbazide to furnish quinazolinylthiourea (38), followed by the cyclization with chloroacetic acid in the presence of anhydrous sodium acetate.
From Thiocyanates and Isothiocyanates:
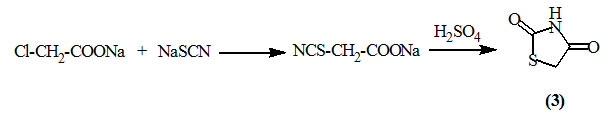
Hendry and Amer, (1958) reported that 2,4-dioxo-thiazolidine (3) was prepared upon treating sodium chloroacetate with sodium thiocyanate to give sodium 2-thiocyanatoacetate then cyclization by sulfuric acid.
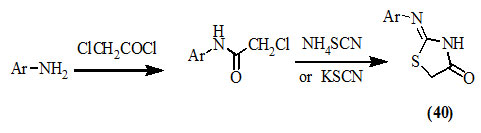
Vicini et al., (2006); Mobinikhaledi et al., (2009); Havrylyuk et al., (2012) reported the synthesis of 2-substitutedimino-4-oxo-thiazolidines (40) by treating the primary amines with chloroacetyl chloride to afford 2-chloro-N-substituted acetamides then hetero-cyclization with ammonium or potassium thiocyanate.
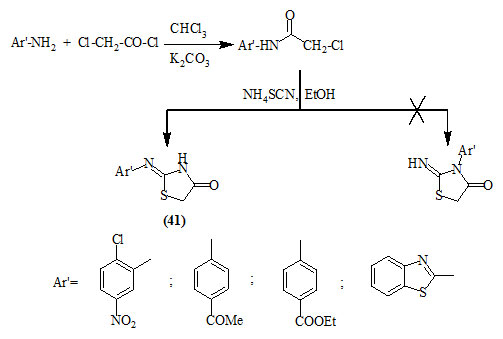
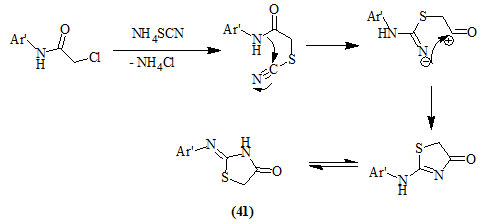
Similarly, Behbehani and Ibrahim, (2012) reported the synthesis of 2-arylimino-4-oxo-thiazolidines (41) from the reaction of aromatic primary amines with chloroacetyl chloride in chloroform/potassium carbonate to give 2-chloro-N-arylacetamide that was cyclized with ammonium thiocyanate in refluxing ethanol.
The formation of compound (41) was explained to take place via the following mechanism:
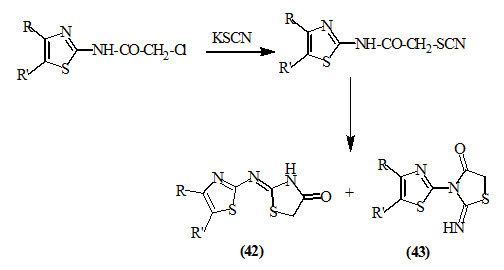
Ostapiuk et al., (2012) reported that the reaction of 2-chloroacetamidothiazole derivatives with potassium thiocyanate gave 4-oxo-thiazolidine derivatives (42) and (43).
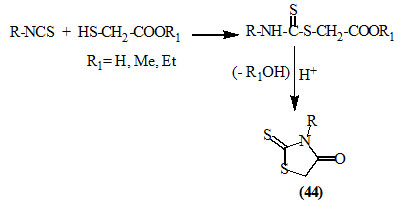
Various 3-substituted-2-thioxo-4-oxo-thiazolidines (44) were conveniently prepared by reacting isothiocyanates with mercaptoacetic acid (thioglycolic acid) or its ester (Drobnica et al., 1972; Ravi et al., 2010).
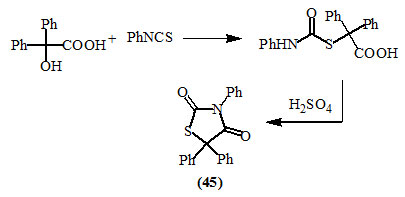
Becker and Bistrzyki, (1919) reported that benzilic acid (in which the electrophilic character of the α-carbon atom is pronounced), reacted with phenyl isothiocyanate to give the respective thiocarbamates which after cyclization by acid yielded 3,5,5-triphenyl-2,4-dioxo-thiazolidine (45).
Imrich et al., (1996) synthesized 3-substituted-2,4-dioxo-thiazolidines (18) by reacting methoxy-N-substituted-iminothiocarbamates, obtained by heating the respective isothiocyanates with sodium methoxide, with bromoacetyl bromide.
El-Gaby et al., (2009) reported that the synthesis of 3-substituted-2-thioxo-4-oxo-thiazolidines (46) was achieved by the cyclocondensation of isothiocyanatosulfonamides with mercaptoacetic acid in the presence of triethylamine (TEA)/dioxane.
From α-Mercaptoalkanoic acids:
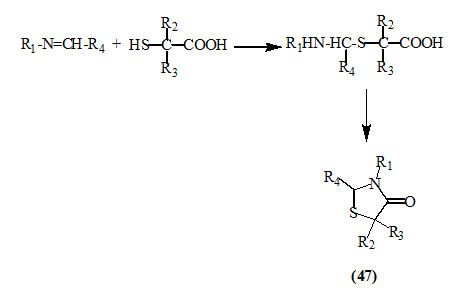
α-Mercaptoalkanoic acids reacted conveniently with various Schiff ‘s bases to give a variety of substituted 4-oxo-thiazolidines (47) (Patel et al., 1976; Soleiman et al., 2005; Desai et al., 2006; Srinivas et al., 2008; Subramanian et al., 2009; Shanmugapandiyan et al., 2010; Akula et al., 2011; Ravichandran et al., 2011; Selvam et al., 2011; Desai et al., 2011; Patel et al., 2012; El-Mekabaty, 2013).
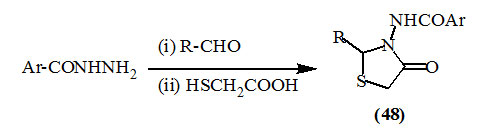
Khalil et al., (2003); Yadav et al., (2005); Mali et al., (2009); Chatrabhuji et al., (2010); Samadhiya et al., (2011); Pane et al., (2012) reported the synthesis of several 2,3-disubstituted-4-oxo-thiazolidines (48) from the condensation of hydrazides with variable aldehydes to give the Schiff’s bases then cyclization with thioglycolic acid under different conditions.
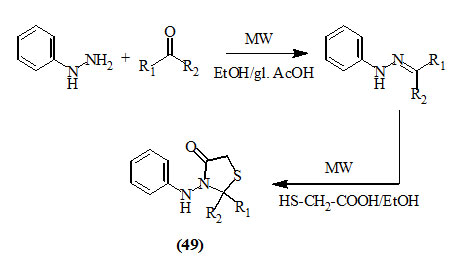
Suthakaran et al., (2008) reported a simple procedure for the preparation of 4-oxo-thiazolidines (49) by using microwave. The synthesis of 2,3-disubstituted-4-oxo-thiazolidines from the reaction of phenylhydrazine with appropriate carbonyl compounds then cyclization with thioglycolic acid in high yield was based upon exposure to microwave irradiation. The reaction rate was enhanced tremendously under microwave irradiation as compared to the conventional method with improved yield.
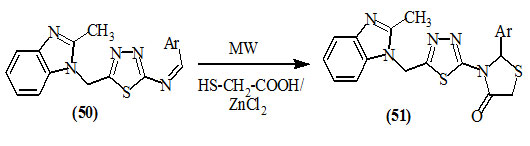
Dua et al., (2010) reported the conventional and greener approach methods for the synthesis of 4-oxo-thiazolidines (51) containing thiadiazole and benzimidazole moieties from the reaction of Schiff base (50) with mercaptoacetic acid in the presence of anhydrous zinc chloride/methanol under microwave irradiation.
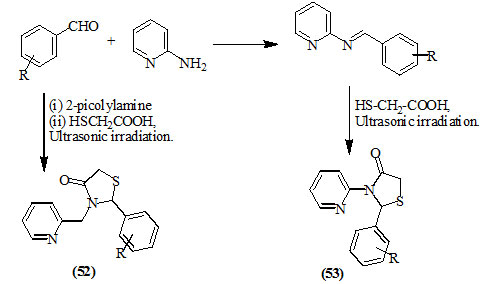
Gouvêa et al., (2012) reported an efficient multi-component synthesis of 4-oxo-thiazolidines (52) and (53) from the reaction of aromatic aldehydes, mercaptoacetic acid and 2-picolylamine or 2-aminopyridine under ultrasound irradiation. Applying the sonochemical methodology, two series of heterocyclic 4-oxo-thiazolidines were synthesized in good yields after short reaction times.
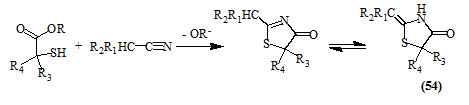
Mercaptoacetic esters reacted smoothly with compounds containing activated nitrile groups in the presence of alcohol to give the respective 4-oxo-thiazolidines (54) (Satzinger et al., 1963).
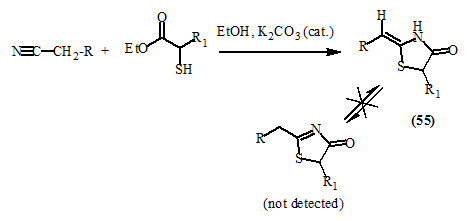
Markovic et al., (2002) reported the stereoselective base-catalyzed reaction affording exclusively (Z)-2-alkylidene-4-oxo-thiazolidine derivatives (55) from the corresponding α-mercaptoesters and activated nitriles in the presence of potassium carbonate as a catalyst and ethanol as a solvent.
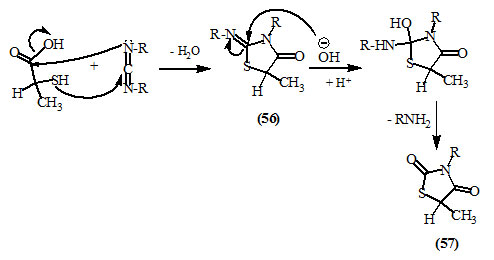
Monforte et al., (1979) synthesized 2-substitutedimino-4-oxo-thiazolidines (56) and 2,4-dioxo-thiazolidines (57) by reacting carbodiimides with α-mercaptopropionic acid.
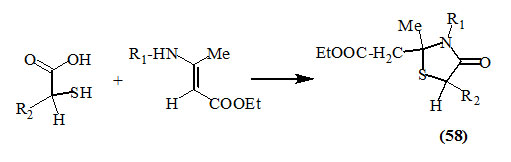
α-Mercaptocarboxylic acids reacted also with enamines derived from ethyl acetoacetate to give the respective 4-oxo-thiazolidines (58) (Klemmensen et al., 1970).
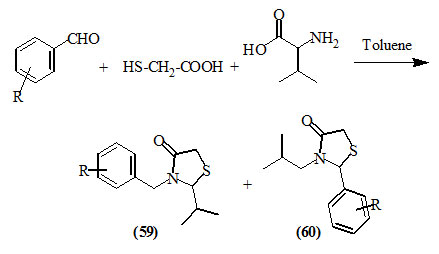
Cunico et al., (2007) reported the one-pot three-component synthesis of 2-isopropyl-3-arylmethyl- (59) and 2-aryl-3-isobutyl- (60) -4-oxo-thiazolidines in good yields, by reacting valine, aromatic aldehydes and mercaptoacetic acid in refluxing toluene.
From trithiocarbonyldiglycolic acid
Strube, (1959) reported that certain 3-substituted-2-thioxo-4-oxo-thiazolidines (44) could be prepared by attacking the carbon atom of the thioxo group of trithiocarbonyldiglycolic acid, with primary amines.
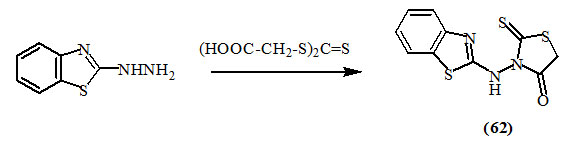
Havrylyuk et al., (2010) reported that the reaction of benzo[d]thiazol-2-yl-hydrazine (61) with trithiocarbonyl-diglycolic acid in refluxing ethanol afforded (62).
From Ketenes
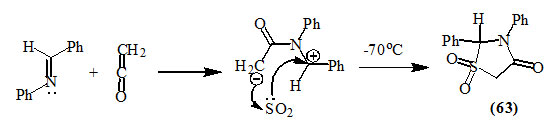
Gomes and Joullie, (1967) reported the formation of 2,3-diphenyl-4-oxo-thiazolidin-1,1-dioxide (63) by the reaction of benzalaniline with ketene in liquid sulfur dioxide at -70oC. The cycloaddition may proceed through a concerted mechanism or the reaction of 1,4-dipole intermediate, formed from ketene and the imine, with sulfur dioxide.
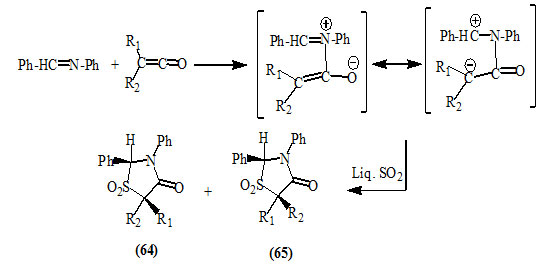
Decazes et al., (1972) studied the cycloaddition of disubstituted ketenes and benzylidine aniline in liquid sulfur dioxide and proposed a two-step ionic mechanism involving dipolar species followed by ring closure to give the diastereomeric 4-oxo-thiazolidin-1,1-dioxides (64) and (65).
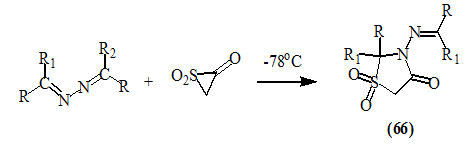
Lysenko and Joullie, (1976) reported that the reactions of both aldehyde and ketone azine derivatives with the ketene-SO2 adduct at -78oC gave 4-oxo-thiazolidin-1,1-dioxide derivatives (66).
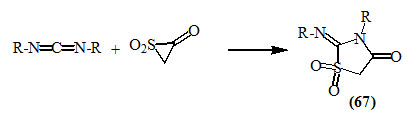
Fisher et al., (1975) studied the cycloaddition reactions of ketene-SO2 adduct to carbodiimide derivatives to afford 2-imino-4-oxo-thiazolidin-1,1-dioxides (67).
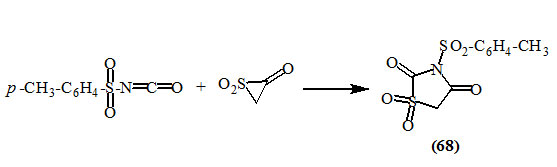
Bohen and Joullie, (1973) studied the reaction of isocyanates with ketene-SO2 adduct. Both aryl and alkyl isocyanates were found to be unreactive. However, the highly reactive p-tolyl-sulphonylisocyanate has been shown to undergo cycloaddition with ketene-SO2 adduct at -10 oC, resulting in the formation of 2,4-dioxo-thiazolidines (68).
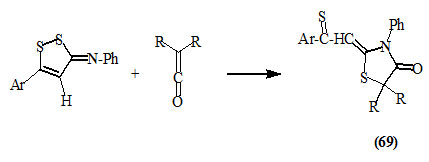
5-Aryl-3-(phenylimino)-1,2-dithioles reacted easily with various ketenes to give 2-(arylthiocarbonylmethylene)-3-phenyl-5,5-disubstituted-4-oxo-thiazolidines (69) (Hervieu et al., 1971).
From Ethyl thiocyanoacetate:
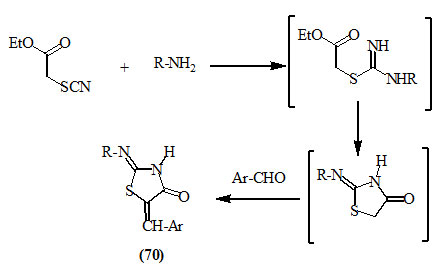
Ethyl thiocyanoacetate reacted with primary amines and aromatic aldehydes to afford 5-arylmethylene-2-substituted imino-4-oxo-thiazolidines (70) (Katritzky and Rees, 1984).
From Thioamides
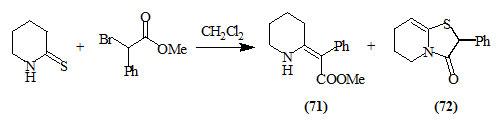
Russowsky and Neto, (2003) reported that the coupling reaction between piperidin-2-thione and methyl 2-bromo-2-phenylacetate in dichloromethane solution afforded the β-enaminocarbonyl compound (71) in 60% yield. In most cases the bicyclic 4-oxo-thiazolidine (72) was produced.
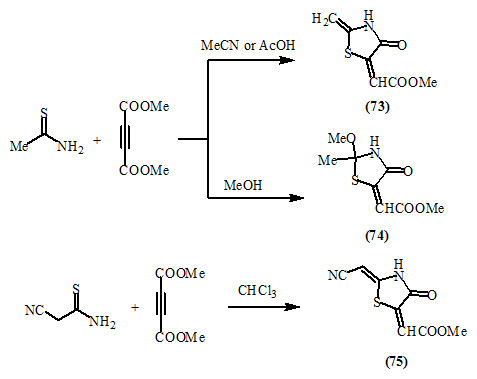
Danikina et al., (2006) reported that the reactions of cyclic and acyclic thioamides with acetylenedicarboxylic esters led to the formation of both acyclic and heterocyclic systems including 4-oxo-thiazolidines (73-75).
From 2-Thiazolines
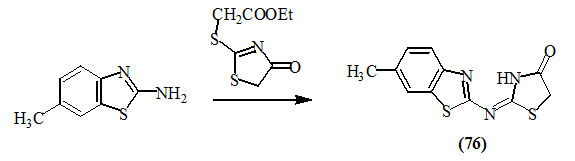
Havrylyuk et al., (2010) synthesized 2-substituted-imino-4-oxo-thiazolidine (76) by reacting 6-methyl-2-aminobenzo-[d]thiazole with 2-ethoxycarbonylmethylthio-4-oxo-2-thiazoline in refluxing ethanol.
Reactions of 4-oxo-thiazolidines
Reactions with acids and bases
Hydrolysis with acids
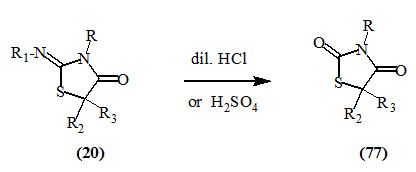
It has been reported that various 2-substituted imino-4-oxo-thiazolidines (20) underwent hydrolysis at position-2 on treatment with dilute hydrochloric acid or sulfuric acid to give the respective 2,4-dioxo-thiazolidines (77) (Aspelund, 1964; Turkevich and Petlichnaya, 1971; Ladnaya and Turkevich, 1973).
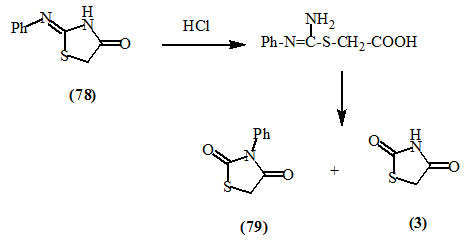
Wheeler and Jamison, (1902) reported that the hydrolysis of 2-phenylimino-4-oxo-thiazolidine (78) with dilute hydrochloric acid afforded a mixture of 2,4-dioxo-thiazolidine (3) and 3-phenyl-2,4-dioxo-thiazolidine (79).
Hydrolysis with alkalis
Andreash, (1879) reported the hydrolysis of 2-imino-4-oxo-thiazolidine (5) when heated with an aqueous solution of barium hydroxide in a sealed tube at 100 oC producing barium mercaptoacetate (80).
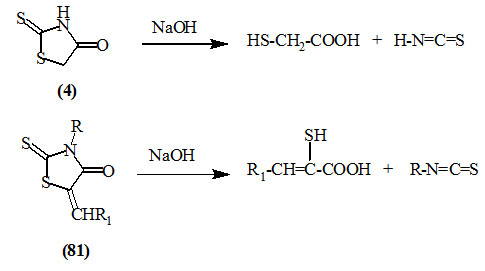
Rhodanine (4) and its alkylidine or arylidine derivatives (81) underwent a ring cleavage (Libermann et al., 1948; Girard and Dreux, 1968) on alkaline hydrolysis to give mercaptoacetic acid and α-mercapto-α,β-unsaturated acids, respectively. Zhou et al., (2007) reported the efficient synthesis of a series of 3-aryl-2-mercaptoacrylic acid derivatives upon the hydrolysis of (81) in alkaline solution under microwave irradiation. Compared with the classical reaction conditions, this method was having the advantages of excellent yields (75~94%), short reaction time (3~6 min), environmental benign, low energy consumption and easy work-up.
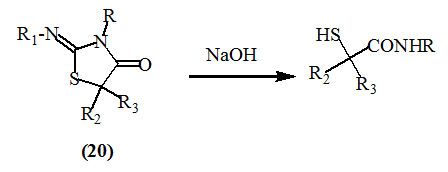
Aspelund, (1964) reported that 2-substituted imino-4-oxo-thiazolidines (20) underwent ring opening to produce the corresponding substituted carbamoylthioglycolic acids upon the treatment with 1N NaOH alone, in alcoholic solution at room temperature, or at reflux temperature.
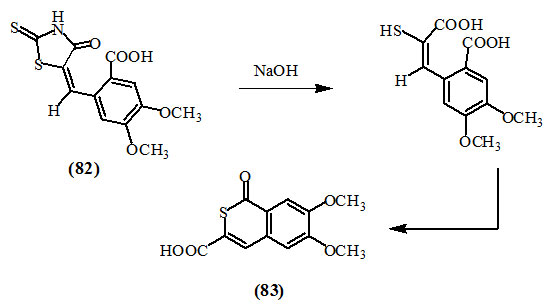
Brown and Newbold, (1952) reported that the treatment of 5-(2-carboxy-4,5-dimethoxyphenylmethylene)-2-thioxo-4-oxo-thiazolidine (82) with 15% sodium hydroxide gave 6,7-dimethoxy-1-oxo-1H-isothiochromen-3-carboxylic acid (83).
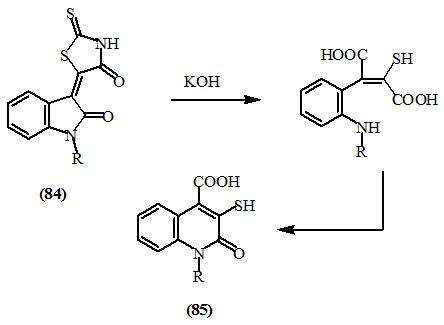
Jones and Henze, (1942) reported that 2-thioxo-4-oxo-thiazolidine derivative of isatin (84) gave, on alkaline hydrolysis, the benzopyridine derivative (85).
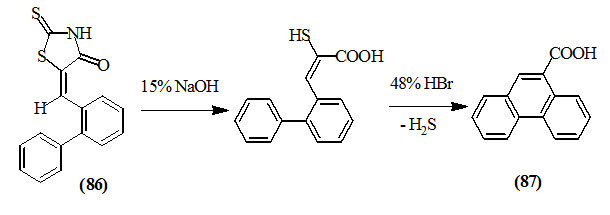
Bradsher and Kittila, (1950) stated that the alkaline hydrolysis of 5-(o-phenylbenzylidene)-2-thioxo-4-oxo-thiazolidine (86) gave α-mercapto-β-(o-phenylphenyl) acrylic acid which was cyclized by refluxing in 48% hydrobromic acid to give 9-phenanthroic acid (87).
Reaction with organic bases
Granacher, (1920) and Mameli, et al., (1954) reported that 2-thioxo-4-oxo-thiazolidine (4) reacted readily with aniline, phenylhydrazine, semicarbazide, or hydroxylamine to afford the respective 2-substituted imino derivative (40).
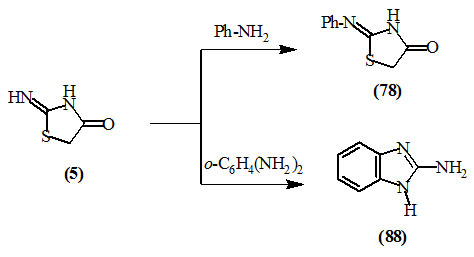
Vorm et al., (1956) reported that 2-phenylimino-4-oxo-thiazolidine (78) and 2-aminobenzimidazole (88) were obtained upon treating 2-imino-4-oxo-thiazolidine (5) with aniline and o-phenylenediamine, respectively.
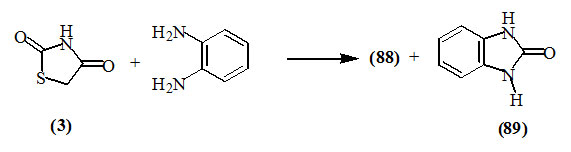
Lipnitskii et al., (1978) reported that o-phenylene-diamine cleaved 2,4-dioxo-thiazolidine (3) and 2-imino-4-oxo-thiazolidine (5) to give the respective benzimidazoles (88) and (89), respectively.
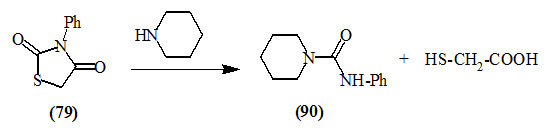
Ruhemann, (1909) reported that the cleavage of 3-phenyl-2,4-dioxo-thiazolidine (79) with piperidine afforded piperidylformanilide (90).
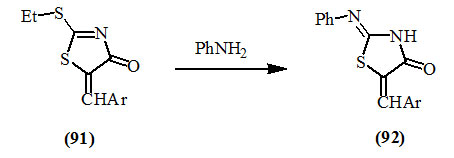
2-Alkylthio-5-arylmethylene-4-oxo-2-thiazolines (91) easily condensed with aniline to give the respective 2-phenylimino-4-oxo-thiazolidines (92) (Ramsh et al., 1975).
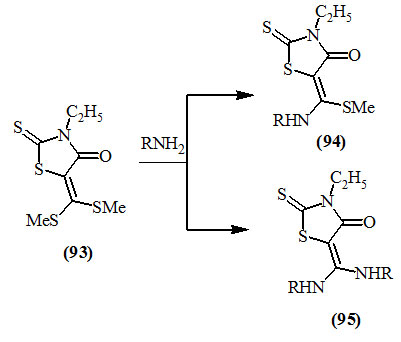
Tominaga et al., (1976) reported that 3-ethyl-5-[bis(metylthio)methylene]-2-thioxo-4-oxo-thiazolidine (93) reacted with nucleophilic reagents such as amines to give the corresponding replacement products of one or two methylthio-group, (94) and (95), respectively in good yields.
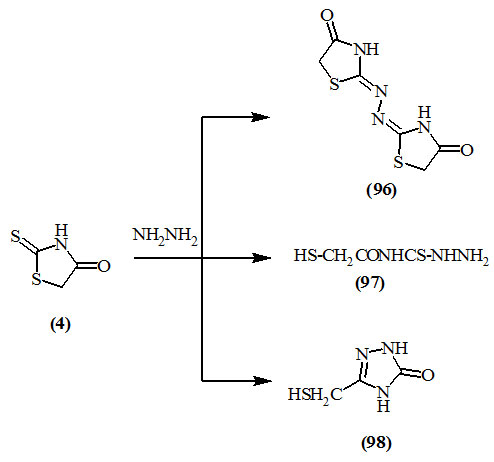
Different accounts dealing with the action of hydrazine hydrate on 2-thioxo-4-oxo-thiazolidine (4) were reported. Thus whereas Taniyama, (1955) reported the formation of the azine derivative (96), Russ, (1961); Davidson, (1964) isolated 4-mercaptoacetyl-thiosemicarbazide (97); and Shvaika et al., (1969) reported the formation of triazolone (98).
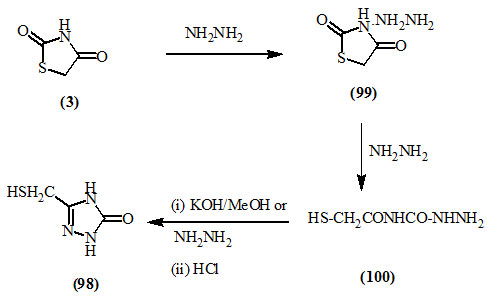
Furthermore, the triazolone (98) was shown to be obtained upon heating 2,4-dioxo-thiazolidine (3) with hydrazine hydrate in ethanol under controlled conditions, it was possible to isolate the reaction intermediates. More detailed information were gained from the work of Shvaika et al., (1970) who reported that hydrazine reacted with 2,4-dioxo-thiazolidine (3) to give 1:1 addition product (99), which underwent ring cleavage to give compound (100). Treatment of (100) with either methanolic potassium hydroxide or hydrazine hydrate then with hydrochloric acid gave the triazolone (98).
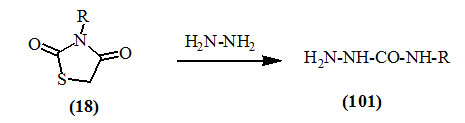
Omar et al., (1980) reported that 3-substituted-2,4-dioxo-thiazolidines (18) were cleaved with hydrazine to give semicarbazide derivatives (101).
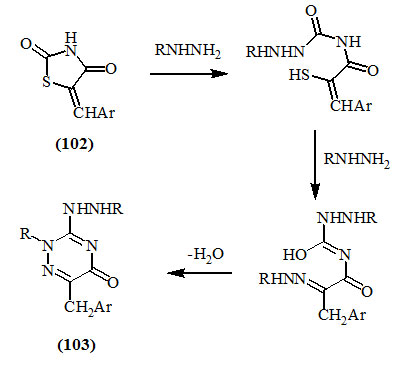
The problem of cleavage of the hetero-ring in 2,4-dioxo-thiazolidines has been extensively studied by Omar et al., (1980; 1981; 1989). They showed that 3-unsubstituted-5-arylmethylene-2,4-dioxo-thiazolidines (102) were cleaved with hydrazines to afford the respective triazinones (103). The route of interconversion was represented by the following scheme:
The 3-substituted derivatives (104), however, afforded the respective biuret derivatives, namely, 1-substituted-amino-3-aryl-5-β-[arylethylidineimino]biuret (105), and the route of interconversion was discussed (Raouf et al., 1975).
The role of the substituents at position-3 on the cleavage of 2,4-dioxo-thiazolidines has been discussed by Omar et al., (1989) and represented as follows:
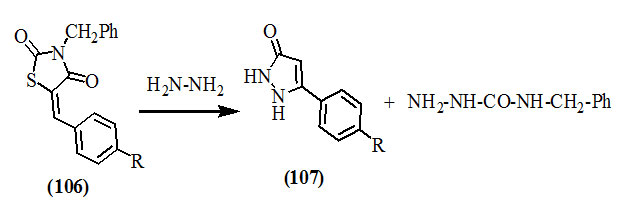
Furthermore, 3-alkylsubstituted-5-arylmethylene-2,4-dioxo-thiazolidines (106) were conveniently transformed into pyrazolinones (107) upon treating with hydrazines, and the route of interconversion has been reported (Omar et al., 1981).
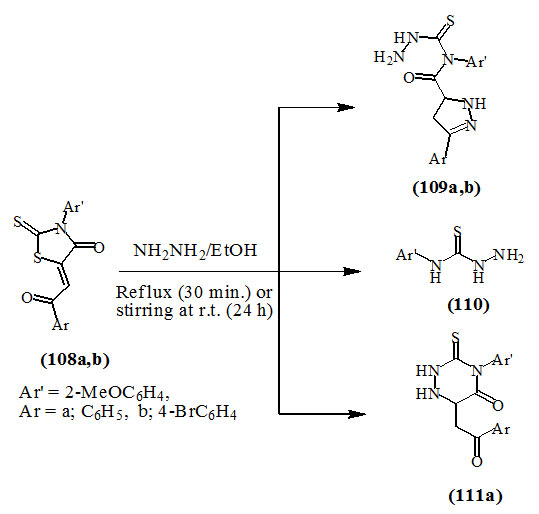
El-Aasar, (2008) reported that the reactions of 3-substituted-5-(2-aryl-2-oxo-ethylidene)-2-thioxo-4-oxo-thiazolidines (108a,b) with 2.5 equiv. of hydrazine were carried out with reflux and/or at room temperature. Both of these conditions gave 4-(3-aryl-4,5-dihydro-1H-pyrazol-5-carbonyl)-4-(2-methoxyphenyl)thiosemicarbazides (109a,b) and 4-(2-methoxy-phenyl)thiosemicarbazide (110). In addition, 6-(2-oxo-2-phenylethyl)-4-(2-methoxyphenyl)-3-thioxo-1,2,4-triazinan-5-one (111a) was obtained from (108a). The successful isolation of sulfur from these reactions was the key to rationalize these transformations.
Subramanian et al., (2009) synthesized pyrazolino-thiazolidines (113) by reacting 5-benzylidene-4-oxo-thiazolidines (112) with phenyl hydrazine in the presence of anhydrous sodium acetate.
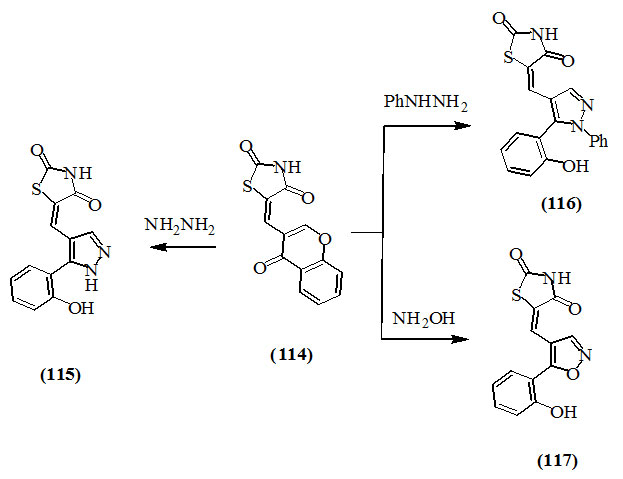
Ibrahim et al., (2011) studied the reaction of 5-[4-oxo-chromen-3-yl)methylene]-2,4-dioxo-thiazolidine (114) with hydrazine, phenylhydrazine and hydroxylamine to produce the corresponding pyrazole and isoxazole derivatives (115-117), respectively.
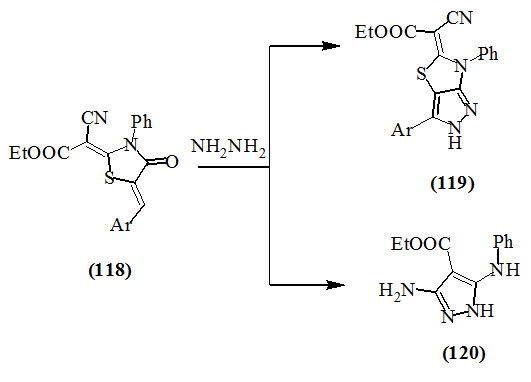
Farhat et al., (2011) reported the conversion of 2,5-diylidene-4-oxo-thiazolidines (118) into pyrazole derivatives (119) and (120) by the reaction with hydrazine.
Reactions depending upon the nucleophilicity of N-3 atom in 3-unsubstituted compounds:
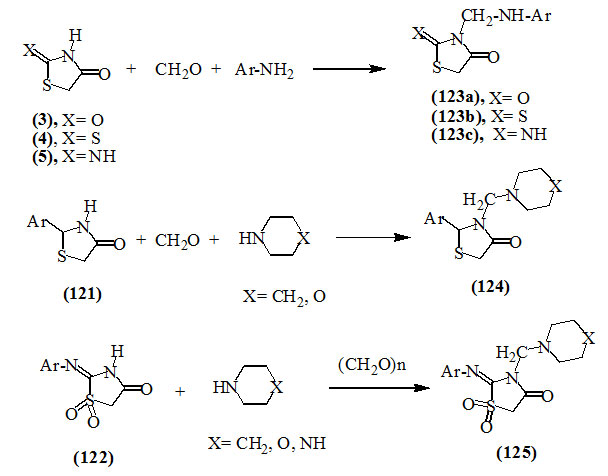
Aminoalkylation reactions (Mannich reaction) of several 4-oxo-thiazolidine derivatives such as 2,4-dioxo- (3), 2-thioxo-4-oxo- (4), 2-imino-4-oxo- (5), 2-aryl-4-oxo- (121), as well as 2-arylimino-1,1-dioxides (122) with formaldehyde or paraformaldehyde and an amine such as aromatic amines or secondary amines were reported to give products (123a-c), (124) and (125) (Hussain and Shukla, 1986; Mohan and Srivastava, 1989; Mahanta et al., 1970; Shi et al., 1991; Wang et al., 1995).
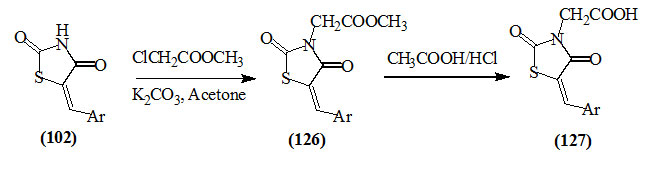
Maccari et al., (2007) reported the reaction of several 5-arylidene-2,4-dioxo-thiazolidines (102) with methyl chloroacetate to give (126) which was hydrolyzed into the acid (127) by HCl/CH3COOH
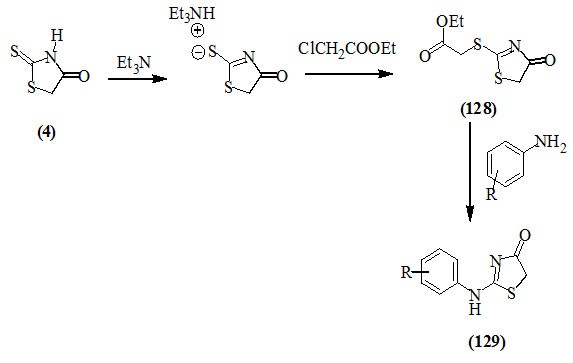
Lesyk et al., (2003) reported that the reaction of 2-thioxo-4-oxo-thiazolidine (4) with ethyl chloroacetate in the presence of triethylamine afforded 2-ethoxycarbonyl-methylthio-4-oxo-2-thiazoline (128). The reaction of (128) with aniline derivatives gave 2-arylamino-4-oxo-2-thiazolines (129) in good yields.
Maccari et al., (2007) stated that the efficient synthesis of 3-substitutedmethyl-5-arylidene-2,4-dioxo-thiazolidine derivatives (130) was occurred by reacting 5-arylidene-2,4-dioxo-thiazolidines (102) with derivatives of methyl bromide in the presence of potassium carbonate and acetone as a solvent, While Kamble et al., (2011) reported a similar reaction in the presence of tetrabutylammonium bromide (TBAB) and sodium hydroxide.
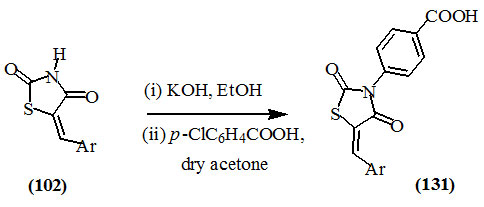
Sonawane and Bari, (2011) reported that the reaction of 5-arylidine-2,4-dioxo-thiazolidines (102) with p-chloro-benzoic acid in the presence of potassium hydroxide as a base and ethanol as a solvent afforded 3-(4-carboxyphenyl)-5-arylidine-2,4-dioxo-thiazolidines (131).
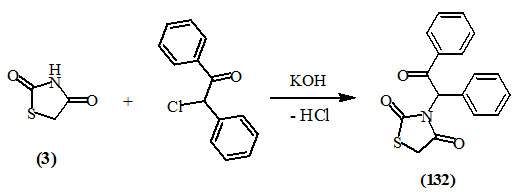
Ali et al., (2007) reported the synthesis of 3-substituted-2,4-dioxo-thiazolidine (132) from the reaction of (3) with 2-chloro-1-oxo-1,2-diphenylethane in the presence of potassium hydroxide.
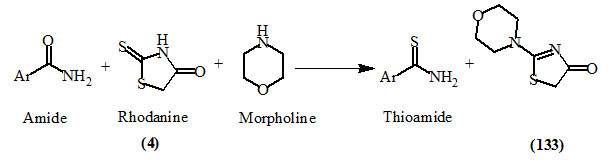
Ray et al., (2013) reported a novel thiation protocol for amide compounds, with the system rhodanine/secondary amine. Clean and efficient synthesis of a variety of thioamides and 4-oxo-2-thiazoline (133) could be achieved through this simple and convenient method using mesoporous silica as an acid catalyst. It was the first report of the use of rhodanine (4) as the potential thiating agent for amide. In this light, this highly efficient catalytic process can be considered as a green technology.
Reactions depending upon the nucleophilicity of the methylene carbanion
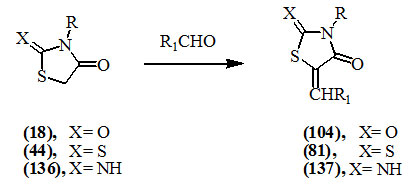
The aldol condensation reaction of the methylene group at position-5 of 2,4-dioxo- (18), 2-thioxo-4-oxo- (44) and 2-imino-4-oxo- (136) thiazolidines with several aldehydes has been widely attempted to give products containing an α,β-unsaturated carbonyl system (104), (81) and (137), respectively.
The condensation between various aldehydes and 2-thioxo-4-oxo-thiazolidine has been affected by microwave irradiation of the reactants adsorbed on a solid phase supporter such as K2CO3/Al2O3 (Zhang et al., 1994; Wang 1995) or KF/Al2O3 (Villemin and Alloum, 1993).
Several aliphatic, aromatic and heterocyclic aldehydes (Prabhakar et al., 1998; Chowdhry et al., 2000; Hu et al., 2002; Tang et al., 2003; Yadav et al., 2005; Vicini et al., 2006; Ibrahim et al., 2007; Irvine et al., 2008; Bhattarai et al., 2009; Kumar and Nanjan, 2010; Wu et al., 2010; Youssef et al., 2010; Ravi et al., 2010; Ibrahim et al., 2011 and Nikalje et al., 2012) and ketones (Brown et al, 1951; Pardasani et al., 2002 and Kodimuthali et al., 2009) have been used. The reaction took place in the presence of ammonia-ammonium chloride (Allen et al., 1952), ethanol/ammonium hydroxide (Brown et al., 1951), sodium ethoxide (Zenno, 1954), mineral acids (Frankevich, 1967), few drops of piperidine (Ruhemann, 1909) and anhydrous sodium acetate/ glacial acetic acid (Giri and Shukla, 1968; Malinnikova et al., 1970; Ladnaya and Turkevich, 1971; Rao and Singh, 1973; Dahl et al., 1974; Achary et al., 1975).
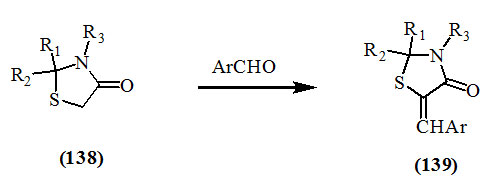
The condensation of aldehydes with 2-alkyl (or aryl)-4-oxo-thiazolidines in the presence of sodium acetate and acetic acid did not occur, possibly because of the decreased reactivity of the methylene groups due to the absence of thioxo or imino groups at position-2. However, the condensation at the methylene group of 4-oxo-thiazolidine (138) had occurred upon using sodium ethoxide in dry benzene (Brown et al., 1963; Snider et al., 1971; Goghari and Parikh, 1977) to afford the respective 5-arylmethylene derivatives (139).
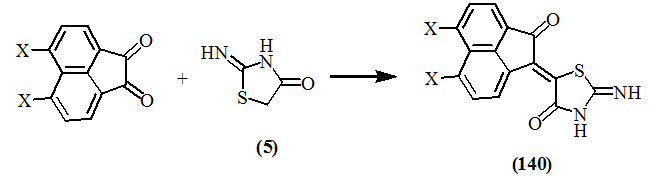
It has been reported that the compounds having more than one carbonyl group underwent condensation with one only of them. Pardasani et al., (2003) reported the formation of (140) upon treating (5) with acenaphthene-quinone or its halogen derivatives.
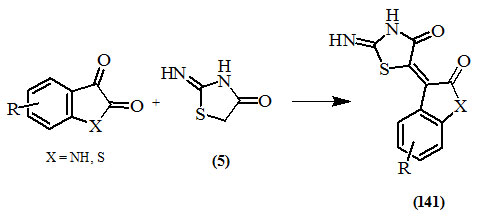
Similarly, the condensation of isatin (indolin-2,3-dione) or benzo[b]thiophen-2,3-dione derivatives with the position-5 of (5) occurred at one of the carbonyl groups affording the 5-arylidine derivatives (141) (Tiwari and Shyam, 1977; Pardasani et al., 2002; Londhe et al., 2005).
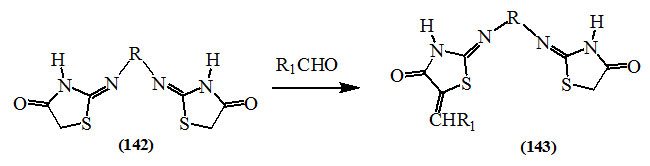
On the other hand, in compounds having more than one active methylene group, the condensation occurred only on one of them. Thus, Achary et al., (1965) reported that the condensation reaction of 4,4′-dioxo-bis(2,2′-substituted imino-thiazolidines) (142) with aldehydes produced the corresponding 5-unsaturated products (143).
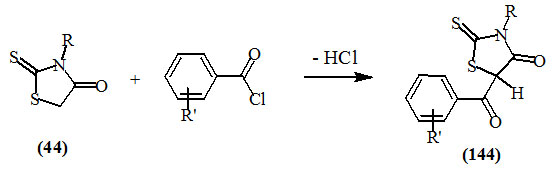
Kvitko et al., (1972) reported that the reaction of substituted benzoyl chlorides with various 2-thioxo-4-oxo-thiazolidines (44) gave 5-aroyl derivatives (144).
Ramsh and Ivanenko, (2003) synthesized 2-imino-5,5-bis(hydroxymethyl)-4-oxo-thiazolidine (145) in 50% yield by treating 2-imino-4-oxo-thiazolidine (5) with formalin in the presence of catalytic amount of triethylamine.
Reactions depending upon the electrophilicity of the exocyclic methylene carbon atom of 5-methylene-4-oxo-thiazolidines:
Reactions with piperidine and thiocresol
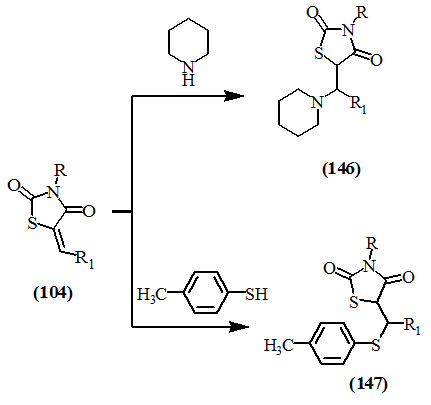
The 1,4-addition to the conjugated carbonyl group has involved attack by anions. Under the proper conditions, 2,4-dioxo-thiazolidnes (104) and piperidine or thiocresol, reacted at room temperature to give (146) and (147), respectively (Mustafa et al., 1960).
Reaction with Grignard reagents
Mustafa et al., (1960) reported that 2,4-dioxo-thiazolidines (104) reacted with organomagnesium halides to give the 1,4-addition products (148).
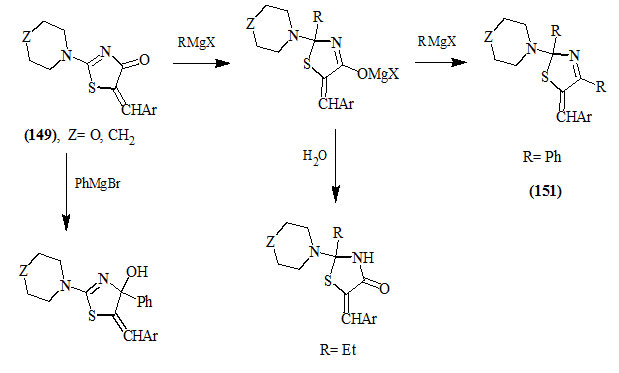
Omar et al., (1981) described the addition of excess ethyl- and phenylmagnesium halide to 5-arylmethyl-ene-2-disubstituted amino-4-oxo-2-thiazolines (149) to give 2-ethyl-4-oxo-thiazolidines (150) and 2,4-diphenyl-3-thiaz-olines (151), respectively. However, the inverse addition of phenylmagnesium bromide to (149) afforded 4-phenyl-2-thiazolin-4-ol derivatives (152).
Reaction with bromine
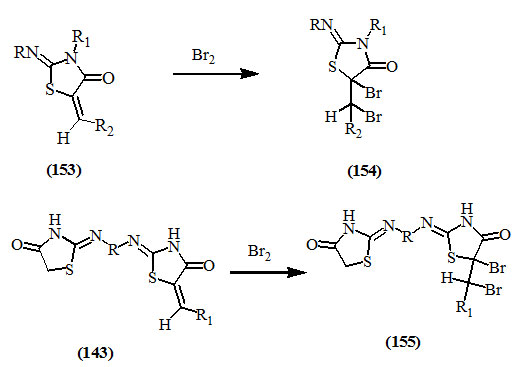
It has been reported that bromine was added to the α,β-unsaturated system of (153) and (143) to produce the respective dibromo compounds (154) and (155) (Pujari, 1966; Chadha et al., 1971; Dahl et al., 1974; Achary et al., 1975).
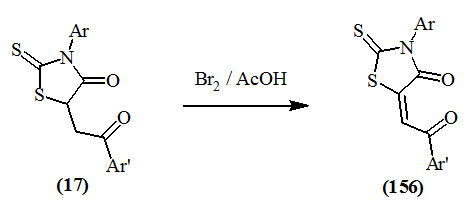
Omar et al., (1995; 2000) and Youssef et al., (2004) reported that 3-aryl-5-(2-aryl-2-oxo-ethyl)-2-thioxo-4-oxo-thiazolidines (17) reacted with bromine in glacial acetic acid solution to afford 5-aroyl-methylene derivatives (156).
Reactions with conjugated dienes
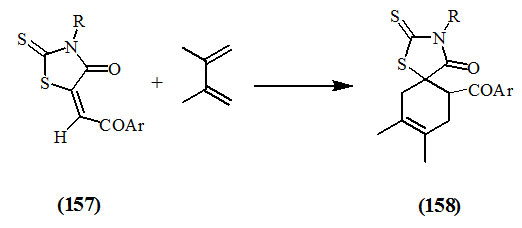
2,3-Dimethylbutadiene reacted at the C=C of 5-aroylmethylene-2-thioxo-4-oxo-thiazolidines (157) to give thiazolidine spiro-cyclohexenes (158) (Nagase, 1974; Omar et al., 1991).
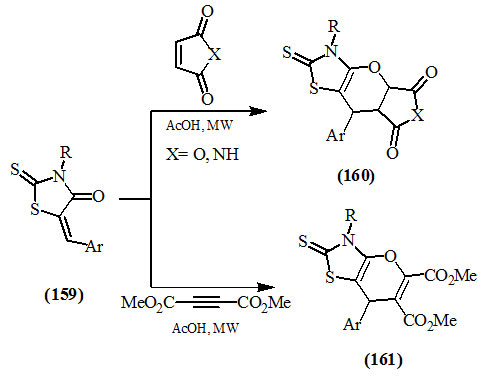
Yarovenko et al., (2008) reported that the cycloaddition reaction of 5-arylidene rhodanine derivatives (159) with the dienophiles, such as maleic anhydride, maleimide and acetylene dicarboxylic ester, under microwave irradiation afforded the Diel’s Alder adducts (160) and (161), within short reaction times and in good yields.
Displacement of hydroxyl group by chlorine
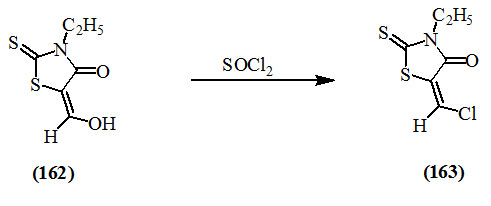
Behringer et al., (1958) reported that thionyl chloride converted 4-hydroxymethylene-3-ethyl-2-thioxo-4-oxo-thiazolidne (162) into 5-chloromethylene-3-ethyl-2-thioxo-4-oxo-thiazolidine (163) which was analog of acid chloride.
Reduction of the exocyclic C=C of 5-arylidene ( or alkylidine) derivatives:
Giles et al., (2000); Zhou et al., (2010) reported the conversion of 5-arylmethylene-2,4-dioxo- (102) and -2-thioxo-4-oxo- (164) thiazolidines to the corresponding 5-arylmethyl derivatives (165) and (166), respectively, by using lithium borohydride in pyridine and tetrahydrofuran. Sodium borohydride and lithium chloride could also be used under these conditions, which represents a cheaper alternative to lithium borohydride.
Friedel-Craft’s reactions
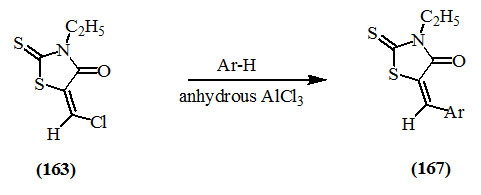
5-Chloromethylene-3-ethyl-2-thioxo-4-oxo-thiazolidine (163) was participated in Friedel-Craft’s reaction with aromatic hydrocarbons to afford the respective 5-arylmethylene derivative (167) (Behringer et al., 1958).
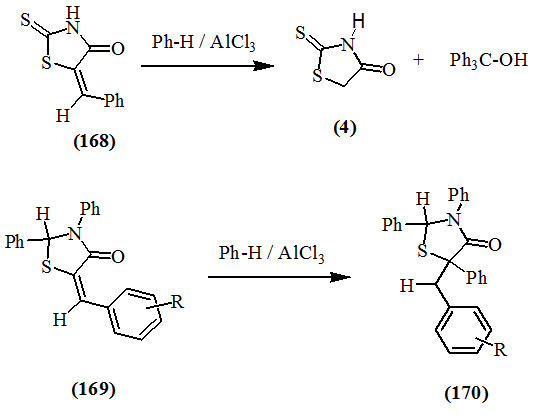
It has been reported that the conversion of 5-benzylidene-2-thioxo-4-oxo-thiazolidine (168) into 2-thioxo-4-oxo-thiazolidine (4) was occurred by reacting with dry benzene in the presence of excess anhydrous aluminum chloride and triphenylcarbinol was isolated. However, 5-arylidene-2,3-diphenyl-4-oxo-thiazolidines (169) under similar conditions added benzene to the unsaturated linkage to give the respective 2,3,5-triphenyl-5-arylmethyl-4-oxo-thiazolidines (170) (Snider et al., 1971).
Oxidation
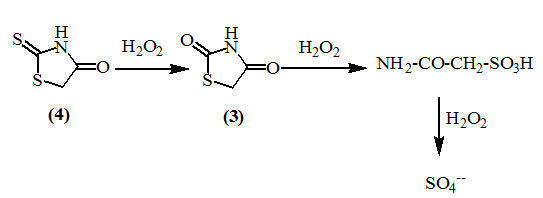
Kitamura, (1938) reported that oxidation of 2-thioxo-4-oxo-thiazolidine (4) with hydrogen peroxide gave 2,4-dioxo-thiazolidine (3). This method was adopted to convert thioxo-group to an oxo-group. Further oxidation of (3) afforded sulfoacetamide which upon further action of the reagent gave the sulfate ion.
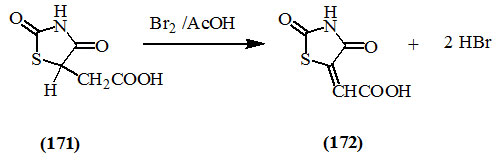
Deghenghi and Daneault, (1960) reported that bromine converted 2,4-dioxo-thiazolidin-5-acetic acid (171) into 5-carboxymethylene-2,4-dioxo-thiazolidine (172).
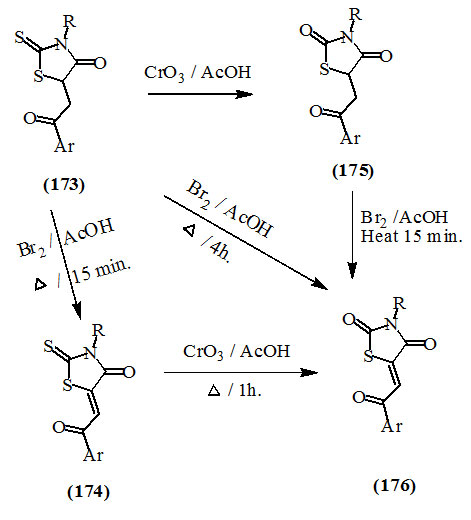
El-Aasar and Saied, (2008) described the oxidation of 5-(2-aryl-2-oxo-ethyl)- (173) and 5-(2-aryl-2-oxo-ethylidene)- (174) 3-aryl-2-thioxo-4-oxo-thiazolidines into the respective 2,4-dioxo-thiazolidine derivatives (175) and (176), respectively via treatment with chromium trioxide in refluxing acetic acid. Also, compound (176) could be obtained from (173) by treating with bromine in glacial acetic acid.
Reactions of 2,4-dioxo-thiazolidin-5-acetic acid:
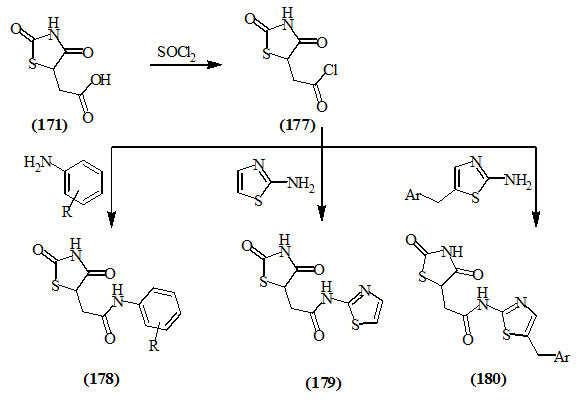
Zimenkovskii et al., (2006) stated that the reaction of 2,4-dioxo-thiazolidin-5-acetic acid (171) with thionyl chloride in refluxing dioxane furnished its chloride derivative (177) that was allowed to react with different amines such as substituted anilines, 2-aminothiazole and 5-arylmethyl-2-aminothiazoles to afford the corresponding amide derivatives (178-180) respectively.
Interconversion of oxo- to thioxo- groups:
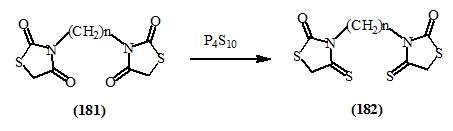
Sulfurization (thiation) of C=O to C=S functions has been affected by treating with tetraphosphorous decasulfide (Orlinsky and Zimenkovsky, 1995) or by Lawesson’s reagent (Cava and Levinson, 1985). With 2,4-dioxo-thiazolidines, thiation has occurred at 4-C=O rather than at 2-C=O group (Orlinsky and Zimenkovsky, 1995). This was exemplified by the conversion of (181) to (182).
Youssef et al., (2002) reported that the thiation of (17) either with tetraphosphorous decasulfide or Lawesson’s reagent in refluxing xylene led to the formation of 3,5-diarylthieno[2,3-d]thiazole-2(3H)thione (183).
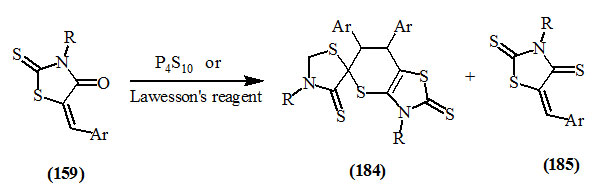
Youssef et al., (2003) reported the thiation of 3-substituted-5-arylidene-2-thioxo-4-oxo-thiazolidine (159) either with tetraphosphorous decasulfide or Lawesson’s reagent in refluxing xylene to afford the compounds (184) and (185).
References
- Abd El-Ghaffar, N. F.; Mohamed, M. A.; Ghanem, H. M.; Zaki, H. M. (2011). J. Am. Sci., 7(4), 771.
- Abrahamson, S.; Westerdahl, A.; Isaksson, G.; Sandstrom, J. (1967). Acta Chim. Scand., 21, 442.
- Achary, T. E.; Dahl, P. N.; Nayak, A. (1975). J. Indian Chem. Soc., 52, 1204.
- Achary, T. E.; Panda, C. S.; Nayak, A. (1965). J. Indian Chem. Soc., 52, 1065.
- Agrawal, V. K.; Sachan, S.; Khadikar, P. V. (2000). Acta Pharm., 50, 281.
- Aibibuli, Z.; Wang, Y.; Tu, H.; Huang, X.; Zhang, A. (2012). Molecules, 17, 3181.
- Akula, G.; Srinivas, B.; Vidyasagar, M.; Kandikonda, S. (2011). Int. J. Pharm. Tech. Res., 3(1), 360.
- Ali, A. M.; Saber, G. E.; Mahfouz, N. M.; El-Gendy, M. A.; Radwan, A. A.; Hamid, M. A. (2007). Arch. Pharm. Res., 30(10), 1186
- Allen, G. G.; Mclean, D.; Newbold, G. T. (1952). J. Chem. Soc., 5053.
- Ameta, U.; Ojha, S.; Bhambi, D.; Talesra, G. L. (2006). Arkivoc, xiii, 83.
- Andreasch, R. (1879). Chem. Ber., 12, 1390.
- Aspelund, H. (1964). Acta Acad. Aboensis, Math. Phys., 24, 23; C. A., 64, 6634a (1966).
- Barlesi, F.; Tchouhadjian, C.; Doddoli, C.; Villani, P.; Greillier, L.; Kleisbauer, J. P.; Thomas, P.; Astoul, P. (2005). Fundam. Clin. Pharmacol., 19, 385.
- Barreca, M. L. (2001). Bioorg. Med. Chem. Lett., 11, 1793.
- Becker, H.; Bistrzyki, A. (1919). Helv. Chem. Acta, 2, 111.
- Behbehani, H.; Ibrahim, H. M. (2012). Molecules, 17, 6362.
- Behringehr, H.; Dillinger, E.; Suter, H.; Kohl, K. (1958). Chem. Ber., 91, 2773.
- Bhattarai, B. R.; Kafle, B.; Hwang, J. -S.; Khadka, D.; Lee, S. -M.; Kang, J. -S.; Hamd, S. W.; Han, I. -O.; Park, H.; Cho, H. (2009). Bioorg. Med. Chem. Lett., 19(21), 6161.
- Blanchet, J.; Zhu, J. (2004). Tetrahedron Lett., 45, 4449.
- Bohen, J. M.; Joullie, M. M. (1973). J. Org. Chem., 38, 2652.
- Bradsher, C. K.; Kittila, R. S. (1950). J. Org. Chem., 15, 374.
- Brown, F. C.; Bradsher, C. K; Bond, S. M.; Potter, M. (1951). J. Am. Chem. Soc., 73, 2357
- Brown, F. C.; Jones, R. S.; Kent, M. (1963). Can. J. Chem., 41, 817.
- Brown, J. J.; Newbold, G. T. (1952). J. Chem. Soc., 4397.
- Capan, G.; Ulusoy, N.; Ergenc, N.; Kiraz, M. (1999). Montsh. Chem., 130, 1399.
- Cava, M. P.; Levinson, M. I. (1985). Tetrahedron, 41(22), 5061.
- Chadha, V. K.; Panda, C. S.; Pujari, H. K. (1971). J. Indian Chem. Soc., 52, 1065.
- Chande, M. S.; Ambhaikar, S. B. (1996). Indian J. Chem., 35B, 373.
- Chande, M. S.; Bhat, U. S. (2000). Indian J. Chem., 39B, 68.
- Chatrabhuji, P. M.; Nimavat, K. S.; Vyas, K. B.; Undavia, N. K. (2010). Res. J. Pharm. Biol. Chem. Sciences, 1(3), 451.
- Chowdhry, M. M.; Mingos, D. M.; White, A. J.; Williams, D. J. (2000). J. Chem. Soc., Perkin Trans. 1, 20, 3495.
- Cunico, W.; Gomes, C. R.; Ferreira, M. D.; Capri, L. R.; Soares. M.; Wardell, S. M. (2007). Tetrahedron Lett., 48, 6217
- Dahl, P. N.; Achary, T. E.; Nayak, A. (1974). J. Indian Chem. Soc., 51, 931.
- Danikina, N. A.; Mikhailov, L. E.; Ivin, B. A. (2006). Russ. J. Org. Chem., 42(6), 783.
- Davidson, J. S. (1964). J. Chem. Soc., 5903.
- Decazes, J. M.; Luche, J. L.; Kagan, H. B.; Partha-Sarthy, R.; Ohrt, J. (1972). Tetrahedron Lett., 3633
- Deghenghi, R.; Daneault, G. (1960). Can. J. Chem., 38, 1255.
- Desai, N. C.; Bhatt, N.; Kumar, M.; Makwana, A. (2011). Indian J. Chem., 50B, 941.
- Diurno, M. V. (1999). Farmaco, 54, 579.
- Drobnica, L.; Knoppova, V.; Komanova, K. (1972). Chem. Zvesti, 26, 538; C. A., 78, 43349y (1973).
- Dua, R.; Sonwane, S. K.; Srivastava, S. K.; Srivastava, S. D. (2010). J. Chem. Pharm. Res., 2(1), 415.
- El-Aasar, N. K. (2008). Al-Azhar Bull. Sci., 19(2), 161.
- El-Aasar, N. K.; Saied, K. F. (2008). J. Sulfur Chem., 29(1), 43.
- El-Gaby, M. S.; Ali, G. A.; El-Maghraby, A. A.; Abdel-Rahman, M. T.; Helal, M. H. (2009). Eur. J. Med. Chem., 44, 4148.
- El-Mekabaty, A. (2013). Int. J. Modern Org. Chem., 2(2), 81.
- Emmet, J. C.; Durant, G. J.; Ganellin, C. R.; Roe, A. M.; Turner, J. L. (1982). J. Med. Chem., 25, 1168.
- Farhat, M. F.; Makhlouf, M. A.; El-Saghier, A. M.; Mezoughi, A. B.; Awhida, S. M.; El-Mehdi, A. A. (2011). Arabian J. Chem., 4, 307.
- Fisher, E.; Hartmann, I.; priebs, H. (1975). Z. Chem., 15, 480; C. A., 84, 150553f (1976).
- Fouli, F. A.; Shaban, M. E.; Youssef, A. S. (1987). J. Prakt. Chem., 329, 203.
- Frankevich, B. S. (1967). Farm. Zh. (Kiev), 22(3), 11; C. A., 68, 29633y (1968).
- Gaikwad, N. J. (2004). Bioorg. Med. Chem., 12, 2151.
- Ganjoo, K. N.; Wakelee, H. (2007). Biologics Targets Therapy, 1, 335
- Gautam, D.; Gautam, P.; Choudhary, R. P. (2012). Chin. Chem. Lett., 23, 1221.
- Giles, R. G., Lewis, N. J., Quick, J. K., Sasse, M. J., Urquhart, M. W.; Youssef, L. (2000). Tetrahedron, 56, 4531.
- Girard, M. L.; Dreux, C. (1968). Bull. Soc. Chim. Fr., 3461.
- Giri, S.; Shukla, S. C. (1968). Indian J. Appl. Chem., 31, 202.
- Goghari, M. H.; Parikh, A. R. (1977). Indian J. Chem., 15B, 17.
- Gomes, A.; Joullie, M. M. (1967). J. Chem. Soc., Chem. Commun., 935.
- Gouvêa, D. P.; Bareňo, V. D.; Bosenbecker, J.; Drawanz, B. B.; Neuenfeldt, P. D.; Siqueira, G. M.; Cunico, W. (2012). Ultrasonics Sonochemistry, 19, 1127.
- Granacher, C. (1920). Helv. Chim. Acta, 3, 152.
- Gupta, R. C.; Nath, R.; Shankar, K.; Bhargava, K. P.; Kishore, K. (1978). J. Indian Chem. Soc., 55, 832.
- Halama, A.; Hejtmànkovà, L.; Lustig, P.; Richter, J.; Srsnova, L.; Jirman, J. (2002). PCT Int. Appl. WO 2002088120.
- Hanusek, J.; Hejtmànkovà, L.; Sterba, V.; Sedlàk, M. (2004). Org. Biomol. Chem., 16, 1756.
- Havrylyuk, D.; Mousla, L.; Zimenkovsky, B.; Vasylenko, O.; Gzella, A.; Lesyk, R. (2010). Eur. J. Med. Chem., 45, 5012.
- Hendry, C. M.; Amer, J. (1958). Chem. Soc., 80, 973.
- Hervieu, P. G.; Rioult, P.; Vialle, J. (1971). Bull. Soc. Chim. Fr., 4380.
- Holmberg, B. (1909). J. Prakt. Chem., 79, 253.
- Hu, B.; Malamas, M.; Ellingboe, J. (2002). Heterocycles, 57, 857.
- Hussain, M. I.; Shukla, S. (1986). Acta Pharm. Jugosl., 36(3), 311; C. A., 106, 131250m (1986).
- Ibrahim, E. S.; Khalil, M. A.; Hassan, A. M.; Abdel-Ghany, Y. S.; Hazzaa, A. A.; El-Sayed, A. E. (2007). Alex. J. Pharm. Sci., 21, 17.
- Ibrahim, M. A.; Abdel-Hamed, M. A.; El-Gohary, N. M. (2011). J. Braz. Chem. Soc., 22(6), 1130.
- Imrich, J.; Bernat, J.; Kristian, P.; Busova, T.; Hocova, S. (1996). Collect. Czech. Chem. Commun., 61(3), 423; C. A., 125, 33524b (1996).
- Irvine, M. W.; Patrick, G. L.; Kewney, J.; Hastings, S. F.; Mackenzie, S. J. (2008). Bioorg. Med. Chem. Lett., 18(6), 2032.
- Ismail, M. F.; Emara, S. A.; Mustafa, O. E. (1991). Phosphorus, Sulfur and Silicon, 63, 373.
- Jones, R. V.; Henze, H. H. (1942). J. Am. Chem. Soc., 64, 1669.
- Kambe, S.; Hayashi, T. (1972). Bull. Soc. Chim. Jpn., 45, 3192.
- Kamble, R. R.; Biradar, D. B.; Meti, G. Y.; Taj, T.; Gireesh, T.; Khazi, I. M.; Vaidyanathan, S. T.; Mohandoss, R.; Sridhar, B.; Parthasarathi, V. (2011). J. Chem. Sci., 123(4), 393.
- Kashkaval, I. T. (1966). Farm. Zh. (Kiev), 21, 9; C. A., 67, 64281h (1967).
- Kashkaval, I. T. (1967). Farm. Zh. (Kiev), 22, 59; C. A., 68, 78191m (1968).
- Katritzky, A. R.; Rees, C. W. (1984). Comprehensive Heterocycl. Chem., 6, 319.
- Khalil, A. A.; Abdel-Hamide, S. G.; Al-Obaid, A. M.; El-Subbagh, H. I. (2003). Arch. Pharm. Pharm. Med. Chem., 2, 95.
- Kitamura, R. (1938). J. Pharm. Sci. Jpn., 58, 8.
- Klemmensen, P. D.; Mortensen, J. Z.; Lawesson, S. O. (1970). Tetrahedron, 26, 4641.
- Kodimuthali, A.; Chary, B. C.; Prasunamba, P. L.; Pal, M. (2009). Tetrahedron Lett., 50, 1618.
- Konenenko, V. E.; Zhitar, B. E.; Baranov, S. N. (1973). Zh. Org. Khim., 9, 61.
- Kvicala, J.; Hovorkova, P.; Paleta, O. (2001). J. Fluor. Chem., 108, 1.
- Kvitko, Y. I.; Bolkhovests, S. V.; Kokurina, A. M. (1972). Khim. Geterotsikl. Soedin., 1491; C. A., 78, 43348x (1973).
- Ladnaya, L. Y.; Turkevich, N. M. (1971). Khim. Geterotsikl. Soedin., 133; C. A., 78, 71977v (1973).
- Ladnaya, L. Y.; Turkevich, N. M. (1973). Farm Zh., 28, 37; C. A., 79, 115484j (1973).
- Laurent, D. R.; Gao, Q.; Wu, D.; Serrano-Wu, M. H. (2004). Tetrahedron Lett., 45, 1907.
- Lesyk, R.; Zimenkovsky, B.; Subtelna, I.; Nektegayev, I.; Kazmirchuk, G. (2003). Acta Polonia Pharm. Drug Res., 60(6), 457.
- Libermann, D.; Himbert, J.; Hengl, J. (1948). Bull. Soc. Chim. Fr., 1120.
- Lipnitskii, V. F.; Shvaika, O. P.; Formenko, V. I.; Baranov, S. N. (1978). Khim. Geterotsikl. Soedin., 666; C. A., 89, 109240s (1978).
- Londhe, A.; Gupta, B.; Khatri, P.; Pardasani, P.; Pardasani, R. T. (2005). Indian J. Heterocycl. Chem., 15, 137.
- Lown, J. W.; Ma, J. C. (1967). Can. J. Chem., 45, 939.
- Lysenko, J.; Joullie, M. M. (1976). J. Org. Chem., 41, 3925.
- Maccari, R.; Ottanà, R.; Ciurleo, R.; Vigorita, M. G.; Rakowitz, D.; Steindl, T.; Langer, T. (2007). Bioorg. Med. Chem. Lett., 17(14), 3886.
- Mahanta, B. C.; Panigrahi, A. H.; Rout, M. K. (1970). J. Inst. Chem., Calcutta, 42, 190.
- Mali, J. R.; Pratap, U. R.; Netankar, P. O.; Mane, P. A. (2009). Tetrahedron Lett., 50, 5025.
- Malinnikova, A. Z.; Chizhevskaya, I. I.; Stremok, I. P. (1970). Sin. Ore. Soedin., 42, 780.
- Mameli, E.; Zorzi, L. (1954). Farmaco (Pavia) Ed. Sci., 9, 691; C. A., 49, 6229b (1955).
- Markovic, R.; Vitnik, Z.; Baranac, M.; Juranic, I. (2002). J. Chem. Res. (S), 485.
- Matar, M. J.; Ostrosky-Zeichner, L.; Paetznick, V. L.; J. R. Rodriguez, E. C.; Rex, J. H. (2003). Antimicrob. Agents Chemother., 47, 1647.
- Mclean, J.; Wilson, F. J. (1939). J. Chem. Soc., 1048.
- Minka, A. F. (1964). Farm. Zh., 19, 47; C. A., 64, 3514c (1966).
- Mobinikhaledi, A.; Foroughifar, N.; Faghihi, S. (2009). Phosphorus, Sulfur and Silicon, 184, 1837.
- Mohan, R. R.; Srivastava, M. (1989). Indian Drugs, 26(7), 342; C. A., 112, 158133m (1990).
- Monforte, P.; Fennech, G.; Basile, M.; Ficarra, P.; Silvestro, A. (1979). J. Heterocycl. Chem., 16, 341.
- Mustafa, A.; Askar, W.; Sobhy, M. E. (1960). J. Am. Chem. Soc., 82, 2597.
- Nagase, H. (1973). Chem. Pharm. Bull., 21, 270, 279.
- Nagase, H. (1974). Chem. Pharm. Bull., 22, 505, 1661.
- Nikalje, A. P.; Choudhari, S.; Une, H. (2012). Eur. J. Exp. Bio., 2(4), 1302.
- Omar, M. T.; Fouli, F. A.; El-Garhi, M. Z. (1991). Bull. Chem. Soc. Jpn., 64, 750.
- Omar, M. T.; Shaban, M. E.; Kasem, M. E. (1981). Ain Shams Sci. Bull., 23, 273.
- Omar, M. T.; Youssef, A. S.; Kandeel, K. A. (1995). Montsh. Chem., 126, 439.
- Omar, M. T.; Youssef, A. S.; Kandeel, K. A. (2000). Phosphorus, Sulfur and Silicon, 162, 25.
- Orlinsky, M. M.; Zimenkovsky, B. S. (1995). Farm. Zh. (Kiev), 4, 64; C. A., 125, 221684s (1996).
- Ostapiuk, Y. V; Obushak, M. D.; Matiychuk, V. S.; Naskrent, M.; Gzella, A. K. (2012). Tetrahedron Lett., 53, 543.
- Pane, A. R.; Sahu, N. U.; Shah, C. P. (2012). Bioorg. Med. Chem. Lett., 22(23), 7131.
- Pardasani, R. T.; Pardasani, P.; Ojha, C. K.; Sherry, D.; Chaturvedi, V.; Sharma, I. (2002). Phosphorus, Sulfur Silicon Relat. Elem. 177, 2435.
- Pardasani, R. T.; Pardasani, P.; Sharma, I.; Saxena, A.; Kohli, S. (2003). Indian J. Chem., 42B, 3075.
- Patel, D. R.; Satpanthi, P. S.; Patel, P. B.; Trivedi, J. J. (1976). J. Inst. Chem., Calcutta, 48, 305.
- Patel, D.; Kumari, P.; Patel, N. (2012). Eur. J. Med. Chem., 48, 354.
- Pfaller, M. A.; Burmeister, L.; Bartlett, M. A.; Rinaldi, M. G. (1988). J. Clin. Microbiol., 26, 1437.
- Polovkovych, S. V.; Karkhut, A. I.; Marintsova, N. G.; Novikov, V. P. (2010). Heteroatom Chem., 21(6), 392.
- Prabhakar, C.; Madhusadan, G.; Sahadev, K.; Maheedhara, R.; Sarma, M. R.; Om, R. G.; Seshagiri, R. C.; Dilip, K. T.; Rajgopalan, R. (1998). Bioorg. Med. Chem. Lett., 8(19), 2725.
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. (2006). BMC Complementary and Alternative Medicine, 1.
- Prakash, G. O.; Anjaneyulu, Y.; Subranian, N. S.; Ramadevi, M.; Vijayalakshmi, G. (2010). Int. J. Chem. Sci., 8(2), 783.
- Ramadan, S. K. and Abou-Elmagd, W. S. I. Synth. Commun. 2018, 48(18), 2409.
- Ramadan, S. K.; El-Helw, E. A. E. J. Chem. Res. 2018, 42, 332.
- Ramadan, S. K.; El-Helw, E. A. E.; Morsy, A. R. United J. Chem. 2018, 1(2), 145.
- Ramadan, S. K.; Hashem, A. I.; Abou-Elmagd, W. S. I.; El-Ziaty, A. K. J. Heterocycl. Chem, 2017, 54, 3711.
- Ramadan, S. K.; El-Ziaty, A. K.; Abou-Elmagd, W. S. I.; Hashem, A. I. Synth. Commun, 2017, 47, 471.
- Ramadan, S. K.; Abou-Elmagd, W. S. I.; El-Ziaty, A. K.; El-Zahar, M. I.; Hashem, A. I. Synth. Commun. 2016, 46(14), 1197.
- Ramadan, S. K.; Hashem, A. I.; Abou-Elmagd, W. S. I.; El-Ziaty, A. K. J. Heterocycl. Chem. 2017, 54, 3711.
- Ramadan, S. K.; Youssef, A. M.; El-Ziaty, A. K.; Abou-Elmagd, W. S. I. J. Heterocyclic Chem. 2015, 52, 278.
- Ramadan, S. K.; El-Ziaty, A. K.; Abou-Elmagd, W. S. I.; Hashem, A. I. Egypt. J. Chem. 2016, 59(4), 637.
- Raman, K.; Pandey, B. R.; Barthwal, J. P.; Bharagava, K. P.; Parmar, S. S. (1978). Res. Commun. Chem. Path. Pharmacol., 21, 177.
- Ramsh, S. M.; Antonova, G. S.; Ginak, A. I.; Sochilin, E. G. (1975). Zh. Org. Khim., 11, 1755.
- Ramsh, S. M.; Ivanenko, A. G. (2003). Chem. Heterocycl. Compd., 39, 1541.
- Ravi, S.; Chiruvella, K. K.; Rajesh, K.; Prabhu, V.; Raghavan, S. C. (2010). Eur. J. Med. Chem., 45, 2748.
- Ravichandran, V.; Jain. A.; Kumar, K. S.; Rajak, H.; Agrawal, R. K. (2011). Chem. Biol. Drug. Des. 78, 464.
- Ray, S.; Bhaumik, A.; Dutta, A.; Butcher, R. J.; Mukhopadhyay, C. (2013). Tetrahedron Lett., 54, 2164.
- Redemann, C. E.; Icke, R.N.; Alles, G.A. (1955). Org. Syn., Coll. Vol. III, 763; John Wiley and sons, Inc., New York.
- Roggero, J.; Audibert, M. (1971). Bull. Soc. Chim. Fr., 4021.
- Ruhemann, S. (1909). Chem. Soc., 95, 117.
- Russ, A. (1961). German Patent, 1, 119, 251; C. A., 57, 668e (1962).
- Russowsky, D.; Neto, B. A. (2003). Tetrahedron Lett., 44, 2923.
- Sahoo, B.; Tripathy, R. B.; Rout, M. K. (1989). J. Indian Chem. Soc., 28B, 358.
- Saiz, C.; Pizzo, C.; Manta, E.; Wipf, P.; Mahler, S. G. (2009). Tetrahedron Lett., 50, 901.
- Samadhia, P.; Sharma, R.; Srivastava, S. K.; Srivastava, S. D. (2011). J. Chem. Sci., 123(5), 631.
- Satzinger, G. (1963). Liebigs Ann. Chem., 665, 150.
- Sedlàk, M.; Hanusek, J.; Hejtmànkovà, L.; Kašparovà, P. (2003). Org. Biomol. Chem., 1, 1204.
- Sedlàk, M.; Hejtmànkovà, L.; Hanusek, J.; Machacek, V. (2002). J. Heterocycl. Chem., 39, 1105.
- Selvam, P.; Radhika, P. P.; Janagaraj, S.; Kumar, A. S. (2011). Research in Biotechnology, 2(3), 50.
- Shanmugapandiyan, P.; Denshing, K. S.; Ilavarasan, R.; Anbalagan, N.; Nirmal, R. (2010). Int. J. Pharm. Sci. and Drug Res., 2(2), 115.
- Shi, H.; Wang, Z.; Shi, H. (1991). Huaxue Tongbao, 8, 36; C. A., 116, 59265g (1992).
- Shvaika, O. P.; Artemov, V. N.; Baranov, S. N. (1970). Khim. Geterotokl. Soedin, 7, 991; C. A., 74, 87899z (1971).
- Shvaika, O. P.; Baranov, S. N.; Artemov, V. N. (1969). Dokl. Akad. Nauk. SSSR., 186, 1102; C. A., 71, 70540r (1969).
- Singh, P.; Ojha, T. N.; Shrama, R. C.; Tiwari, S. (1994). Indian J. Pharm. Sci., 57, 162.
- Singh, S.; Yadav, A.; Meena, A. K.; Singh, U.; Singh, B.; Gaurav, A.; Rao, M. M.; Panda, P.; Singh, R. (2010). Int. J. Chem. Anal. Sci., 1(5), 79.
- Snider, R. H.; Beale, L. M.; Gaillard, R.; Coggon, P.; Mcphail, A. T.; Brown, F. C. (1971). Int. J. Sulfur Chem., 1, 191.
- Soleiman, H. A.; Koraiem, A. I.; Mahmoud, N. Y. (2005). J. Chin. Chem. Soc., 52, 119.
- Sonawane, L. V.; Bari, S. B. (2011). Int. J. Biol. Chem., 5(1), 68.
- Stephen, H. W.; Wilson, F. J. (1926). J. Chem. Soc., 2531.
- Strube, R. E. (1959). Org. Synthesis, 39, 1.
- Subramanian, N. S.; Omprakash, G.; Anjaneyulu, Y.; Gupta, V. R.; Ramadevi, M. (2009). Int. J. Chem. Sci., 7(3), 1537.
- Suthakaran, R.; Kavimani, S.; Venkappayya, D.; Jayasree, P.; Deethi, V.; Tehseen, F.; Suganthi, K. (2008). Rasayan J. Chem., 1(1), 30.
- Svetkin, Y. V.; Minlibaeva, A. N.; Vasileva, S. A. (1968). Zh. Obshch. Khim., 38, 116; C. A., 69, 59141z (1968).
- Tang, E.; Yang, G.; Yin, J. (2003). Spectrochimica Acta part A 59, 651.
- Taniyama, H.; Yasui, B. (1955). J. Pharm. Soc., Jpn., 75, 200; C. A., 50, 1777h (1956).
- Tiwari, I. C.; Shyam, R. (1977). Curr. Sci., 46, 619.
- Tominaga, Y.; Sone, M.; Mizuyama, K.; Matsuda, Y.; Kobayashi, G. (1976). Chem. Pharm. Bull., 24, 1671.
- Toubal, K.; Djafri, A.; Chouaih, A.; Talbi, A. (2012). Molecules, 17, 3501.
- Trivedi, J. P.; Contractor, S. J.; Shah, I. D. (1966). J. Indian Chem. Soc., 43, 265.
- Turkevich, N. M.; Petlichnaya, L. I. (1971). Metody Poluch. Khim. Reaktiv. Prep., 23, 13; C. A., 78, 16092c (1973).
- Vàňa, J.; Hanusek, J.; Ržička, A.; Sedlàk, M. (2009). J. Heterocycl. Chem., 46, 635.
- Vicini, P.; Geronikaki, A.; Anastasia, K.; Incerti, M.; Zani, F. (2006). Bioorg. Med. Chem., 14(11), 3859.
- Villemin, D.; Alloum, M. (1993). Phosphorus, Sulfur, Silicon Relat. Elem., 79, 23; C. A., 120, 134356a (1994).
- Walczynski, K.; Timmerman, H.; Zuiderveld, O. P.; Zhang, M. Q.; Glinka, R. (1999). Farmaco, 54, 533.
- Wang, G. (1995). Huaxue Shiji, 17(1), 51; C. A., 123, 55453p (1996).
- Wheeler, H. I.; Jamison, G. S. (1902). J. Am. Chem. Soc., 25, 680.
- Wu, Y.; Tai, H. H.; Cho, H. (2010). Bioorg. Med. Chem. Lett., 18(4), 1428.
- Yadav, R.; Srivastava, S. D.; Srivastava, S. K. (2005). Indian J. Chem., 44B, 1262.
- Yarovenko, V. N.; Nikitina, A. S.; Zayakin, E. S.; Zavarzin, I.; Krayushkin, M. M.; Kovalenko, L. V. (2008). Arkivoc, iv, 103.
- Youssef, A. M.; Omar, M. T.; El-Khamry, A.; Ramadan, S. (2002). Sulfur Letters, 25(4), 173.
- Youssef, A. M.; Omar, M. T.; El-Khamry, A.; Ramadan, S. (2003). Phosphorus, Sulfur and Silicon, 178, 1.
- Youssef, A. M.; Omar, M. T.; Nessim, M. I. (2004). Egypt J. Chem., 47(1), 75.
- Youssef, A. M.; White, M. S.; Villanueva, E. B.; El-Ashmawy, I. M.; Kleqeris, A. (2010). Bioorg. Med. Chem., 18(5), 2019.
- Zenno, H. (1954). J. Pharm. Soc. Jpn., 74, 199.
- Zhang, M.; Wang, C.; Yu, S.; Tian, Z.; Zhang, L.; Chen, Z.; Li, J. (1994). Gaodeng Xuexiao, Huaxue Xuebao, 15(11), 1647; C. A., 122, 213992z (1995).
- Zhou, C.; Tang, C.; Chang, E.; Ge, M.; Lin, S.; Cline, E.; Tan, C. P.; Feng, Y.; Zhou, Y. P.; Eiermann, G. J.; Petrov, A.; Salituro, G.; Meinke, P.; Mosley, R.; Akiyama, T. E.; Einstein, M.; Kumar, S.; Berger, J.; Howard, A. D.; Thornberry, N.; Mills, S. G.; Yang, L. (2010). Bioorg. Med. Chem. Lett., 20(3), 1298.
- Zimenkovskii, B. S.; Kutsyk, R. V.; Lesyk, R. B.; Matyichuk, V. S.; Obushak, N. D.; Klyufinska, T. I. (2006). Pharm. Chem. J., 40(6), 13.