Introduction
Hydrogen–hydrocarbon fuels have been engaging the attention as promising fuels for power generation applications for the following reasons. The first reason relates to the opportunity of adding hydrogen to methane in order to extend operability ranges of a combustion device, to reduce NOx emissions and to take advantage of lean combustion in both stationary [1] and mobile [2] systems. The second reason is closely linked to the global warming and the perspective of using hydrogen in both fuel cells and combustion devices [3].
In addition, lean premixed combustion is one of the means to meet the increasingly strict NOx emission regulations for power devices for energy generation and transportation [4, 5]. Reduction of flame temperature provided by using lean combustion can markedly reduce NOx generation, but additional investigations are required to provide wider implementation of the technology. However, many problems remain unresolved for the wider use of lean premixed combustion. Lower temperatures of lean premixed combustion can lead to suppression of oxidation reactions, increasing CO and decreasing stability operation of a combustor [2].
Being composed mainly of methane, natural gases usually contain other gases from a few percent up to as much as 18%, depending on the deposit [2, 6]. These impurities are usually ethane, propane and butane. Composition variations in natural gases can have a noticeable impact on the combustion chemistry, emissions composition, and stability.
Yet it is precisely for the lean fuel gas mixtures, catalytic ignition can show its prominent advantages [7]. With catalyst, it is possible to ignite leaner mixtures without the conventional spark plug. There are no suppressing effects as when using spark plug electrodes and one can place the ignition source arbitrarily in the combustion cylinder. Catalytic ignition needs no spark electrodes, therefore no erosion effects occur and, consequently, the ignition system can operate significantly longer than that that with a spark plug.
There is a need to work out the catalysts that perform oxidation at comparably low temperatures (< 250 °C), especially to increase the efficiency of combustion devices [8]. The features of stability of the catalyst operation have been investigated [9]. Palladium catalysts supported on alumina have poor stability for methane conversion. By adding platinum to the catalysts a considerably higher stability is achieved. The Pd–Pt catalysts, on the other hand, are inhibited by water. After removal of water, the activity is almost fully recovered. The drop of conversion over Pd/Al2O3 is not seen for all fuels that produce water during combustion. Hydrogen presents a very stable conversion over Pd/Al2O3. In contrast, the conversion of ethane is slightly decreasing with time, but not to the large extent observed during methane conversion.
Noble metals impact on the flammability of hydrogen–methane blends in different ways. It was shown that the ignition temperature of the mixture 40%H2 – air over Pd metal (700C, 1 atm) is ~ 2000 less than over the Pt surface (2600C, 1 atm) [10, 11]. In addition, Pd ignites stoichiometric mixes (30÷60% H2 + 70÷40%CH4) + air (f = 1, equivalence ratio f is a fraction of fuel in the mix with air: fH2 + 0.5 (O2 + 3.76N2)); metallic Pt cannot ignite these up to 4500C, i.e. Pd is more effective than Pt. It was also shown that the cellular structure of a flame front at ignition with Pd is not observed as compared with the results obtained over Pt surface. Thus, Pd seems to be more usable for hydrogen recombinators in NPP, because no catalytic particles as ignition centers formed by decomposition of volatile oxide can appear in gas phase as compared to Pt [12]. The experimental value of the effective activation energy of the process is estimated as 3.5±1 kcal/mole that is characteristic of surface processes. It indicates the noticeable role of the dark reaction of consumption of H2 and O2 observed directly at low pressures [10]. The occurrence of that reaction reduces the probability of accidental explosion as compared with Pt.
The present paper concentrates on the combustion of hydrogen- hydrocarbon (C1 – C6, namely CH4, C2H6, C3H8, C4H10, C5H12, C6H14) – air mixtures with f = 0.6÷1.2 over Pd at total pressures 1÷2 atm. The paper is aimed at an establishment of both features of a flame front propagation in the mixtures and the dependence of a flammability limit over Pd surface on temperature.
Experimental
The experiments were performed with gas mixtures of 30% C1 – C6 hydrocarbon + 70%H2 + air at f = 0.6÷1.2, and pressures 1÷2 atm. A heated cylindrical stainless steel reactor 25 cm in length and 14 cm in diameter, equipped with demountable covers and an optical sapphire window in one of the covers was used in experiments (Fig.1). The accuracy of temperature measurements was 0.3K. Registration of ignition and flame propagation was performed by means of a color high-speed camera Casio Exilim F1 Pro (frame frequency –600 s-1). A video file was stored in computer memory and its time-lapse processing was performed [12]. The pumped and heated reactor was quickly filled with the gas mixture from a high-pressure buffer volume with the necessary pressure. The ignition limit was determined as the mean of two temperatures at the given pressure; at higher temperature the ignition occurred, at a lower one the ignition was missing. An electromagnetic valve was used to open and close gas communications. A pressure transducer recorded pressure in the course of gas intake and combustion. A Pd coiled wire 70 mm long, and 0.3 mm in diameter was placed in the reactor. The Pd wire was used both to ignite the flammable mix and to estimate the warming-up of the wire as a bridge arm. Before each experiment, the reactor was pumped down to 0.01 Torr; after each ignition, the pumping continued during 1.5 hours to pump out most of the water vapor. Total pressure in the reactor was monitored with a vacuum gauge, and the pressure in the buffer volume was controlled with a manometer. Chemically pure gases and 99.85% Pd were used.
Results and discussion
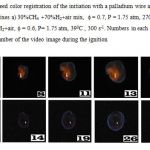
The typical frame sequences of the spatial development of ignition with Pd wire and flame propagation of preliminary prepared mixes 30%CH4 +70%H2+air and 30%C2H6 +70%H2+air at f = 0.6÷0.9, and pressures 1.75 atm are presented in Fig.2 a,b. Just like in case of Pt [10-12], Pd wire becomes red-hot before and after ignition due to catalytic reactions on the Pd surface. As is seen, in lean mixes, the cellular structure of the flame front is observed: the thermo diffusive instability of fuel lean flames leads to the flames that form well-known cellular structures, going back to early work by Markstein [13] and Zeldovich [14].
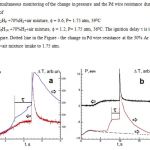
In Fig.3, the results of simultaneous registration of the change in pressure and the change in Pd resistance (proportional to the warming-up) during the ignition and P= 1.75 atm of mixes a) 30%C2H6 +70%H2+air mixture, f = 0.6, 390C and b) 30%C6H14 +70%H2+air mixture, f = 1.2, 380C are shown. Because the Pd foil is not heated up uniformly [12] (see also Fig. 2 a,b, frames 1, 2), therefore, the time dependence of resistance, which represents arbitrary temperature, is somewhat delayed as compared with filming. The break on that dependence corresponds to the resistance at the moment of ignition. Evidently, the temperature value measured by means of Pd wire is a lower boundary of the real temperature of the ignition center, which ignites the combustible mix. Indeed, it takes a certain time to warm up the wire, therefore, if one obtains the values of the temperature by the method, these will be underestimated. The dotted line in Fig.2 is the change in Pd wire resistance at the 30% Ar + 70%H2 + air mixture intake. Thus, the first maximum in the dependence of the resistance during combustion is not due to the ignition process, but only to the H2 interaction with Pd.
As is seen in Fig.3, total pressure in the reactor reaches 1.75 atm prior to the moment of the ignition, i.e. the ignition occurs upon completion of gas input in cases a) and b). Therefore, the period of ignition delay t for 30%C2H6 + 70%H2 + air mixture makes ~ 2 s; it makes 8 s at 240C and P = 1 atm. Thus, the fuel can be ignited with Pd wire at room temperature, evidently without external energy supply. The 30%C2H6 + 70%H2 + air mix f=0.6 shows the lowest temperature of the ignition limit: 230C at 1 atm.
We have found that the mixes 30%CH4 +70%H2+air and 30%C3H8 +70%H2+air manifest two values of the temperature ignition limit. The higher one can be achieved with a bottom-up approach by temperature, the lower one is attained in the processing of the reactor with ignitions. That is illustrated by the dependencies of the flammability of the mixes 70%CH4 +30%H2+air (Fig.4a) and 30%C3H8 + 70%H2 + air (Fig.4b) on the number of successive ignitions at P= 1.75 atm. As is seen in the Figure, the temperature of ignition limit in the “fresh” reactor (a bottom-up approach: no ignitions occurred before) is ~ 3150C at f=0.9. At this temperature the mixes with φ<0.9 at the same pressure do not ignite in the “fresh” reactor. However, during processing with ignitions, the temperature of ignition limit markedly drops and makes 2740C for φ=0.7 after 7 ignitions. It was shown that the process is reversible: after reactor treatment with O2 the ignition limit returns to its initial value ~ 3150C. Similar dependencies were observed during ignitions of the mix 30%C3H8 + 70%H2 + air (Fig.4b). The temperature of ignition limit in the “fresh” reactor is ~ 1080C at f=1. During further ignitions of the same mix, the temperature of ignition limit decreases and makes 300C after 7 ignitions. The process is also reversible: after reactor treatment with O2 the ignition limit returns to its initial value ~ 1080C. Thus, the phenomenon observed is of a hysteresis nature; this can be due to reversible changes in Pd surface and hence in the activity of the catalyst. The authors [15] claim that they observed hysteresis phenomena at oxidation of methane over palladium foil under flow conditions, however, no data had been provided.
However, the changes in Pd surface are pronounced only for H2 –methane and H2 – propane blends; for the other blends the hysteresis effect is missing. It means that the ignition limit over Pd is determined also by features of the kinetic mechanism of hydrocarbon oxidation. The ignition limits for these blends at total pressure 1.75 atm are presented in the Table 1.
Table 1: Ignition limits for blends 70%H2 + 30% (C2, C4-C6) at 1.75 atm
| Blend | 30%C2H6 +70%H2 φ = 0.6 | 30%C4H10 +70%H2 φ = 1.1 | 30%C5H12 +70%H2 φ = 1.2 | 30%C6H14 +70%H2 φ = 1.2 |
| Temperature at the ignition limit, 0C | 20 | 28 | 24 | 36 |
To estimate the effective activation energy of the overall reaction for the blends, which do not exhibit features due to reversible changes in catalyst activity, the temperature dependences of the delay periods of ignition were examined. The experimental dependences of the delay periods of ignition t on temperature in Arrhenius coordinates for 30% (C2H6, C4H10, C5H12, C6H14) + 70%H2 + air mixes are shown in Fig. 5. As is seen in Fig.5, the dependences can be approximated by a straight line (the correlation coefficient is 0.98). The data were processed with the use of the program package Statistica 9 (Statsoft). From Fig. 5, the experimental value of effective activation energy of the processes is E = 2.4±1 kcal/mole that is characteristic of a surface process [16]. This value is very close to one estimated in [11] from the dependence of the H2 fraction in H2 – air and H2 + CH4 + air mixtures on temperature (3.5±1 kcal/mole). From that we can conclude that the dependence for mixes 30%(C2, C4, C5, C6) + 70%H2 + air is determined with the only H2 fraction in the mixture as it was shown for H2 – air and H2 – CH4 – air mixes in [11]. This might indicate that the values of effective activation energy shall be from the same process, probably the branching one [11]. It means that the branching step has probably heterogeneous nature. Really, to provide the ignition, the cycle of reactions, in which the branching (an increase in the number of active centers) takes place, must occur [17]. The branching rate can change e.g. at the expense of the additional branching step. The step can be probably H + HO2 → 2 OH, in which a relatively inactive HO2 turns into active OH, i.e. branching in addition to the step H + O2 → O + OH occurs. As is shown in [17] accounting for the reaction H + HO2 allows explaining an extension of the ignition area in the presence of external H atoms. In our case the source of the atoms is a chain initiation step H2 + O2 → 2 OH; its rate increases in the presence of a catalytic wire.
 |
Fig.5 Arrhenius plots of the experimental dependences of the delay periods of ignition on temperature at P=1.75 atm.
Filled circles – 30%C2H6 +70%H2+Air at φ=0.6; Empty circles – 30%C4H10 +70%H2+Air at φ=1.1; Triangles – 30%C5H12 +70%H2+Air at φ=1.2; Thick circles – 30%C6H14 +70%H2+Air at φ=1.2 |
In addition, the lowest temperature of the ignition limit of hydrogen -air mix over Pd surface is ~ 700C for 40% H2 + 60% air [11]. As the ignition limit temperature for mixes of 70% hydrogen + 30% hydrocarbon (C2-C6) + air is ~ 400 lower than that value (see Table 1), it indicates an important role of the reactions with the participation of hydrocarbon molecules on palladium surface.
Conclusions
It was experimentally shown that the temperature of the ignition limit over Pd at P=1.75 atm, measured with a bottom-up approach by temperature, of the mixtures 30% methane + 70% hydrogen + air (f=0.9, T=317oC) and 30% propane + 70%H2 + air (f=1, T=106oC) markedly drops after subsequent ignitions to T=270oC for H2 – CH4 mix and to T = 320 C for the H2 – C3H8 blend. The ignition limit returns to the initial value after treatment of the reactor with O2 or the air, i.e. a hysteresis phenomenon occurs. The ignition limit of the mixtures 30% (C2, C4, C5, C6) + 70%H2 + air (f=0.6, 1.1, 1.2, 1.2, correspondingly) over Pd amounts to 25÷35 oC at P=1.75 atm; the hysteresis effect is missing. It was found that the lean 30%C2H6 + 70%H2 + air mix (f=0.6) shows the lowest temperature of the ignition limit: 240C at 1 atm. The estimate of the effective activation energy of the ignition of the mixes over Pd is ~ 2.4 ± 1 kcal/mole that is characteristic of a surface process. Thus, the usage of Pd catalyst allows igniting H2-hydrocarbon mixtures at 1÷2 atm at initial room temperature without external energy sources.
References
- Tang C, Zhang Y, Huang Z. Progress in combustion investigations of hydrogen enriched hydrocarbons, Renewable and Sustainable Energy Reviews 30: 195–216. 2014.
- Knyazkov A, Shvartsberg VM, Dmitriev AM, Osipova KN, Shmakov AG, Korobeinichev OP, Burluka A Combustion Chemistry of Ternary Blends of Hydrogen and C1–C4 Hydrocarbons at Atmospheric Pressure, Combustion, Explosion, and Shock Waves. 53: 491–499. 2017.
- Biswas S, Tanvir S, Wang, H, Qiao L On ignition mechanisms of premixed CH4/air and H2/air using a hot turbulent jet generated by pre-chamber combustion, Applied Thermal Engineering. 106: 925–937.2016.
- Cho E.-S and Chung, SH Improvement of flame stability and NOx reduction in hydrogen-added ultralean premixed combustion, Journal of Mechanical Science and Technology. 23: 650-658. 2009.
- Razali H, Sopian K, Mat S Green fuel: 34% reduction of hydrocarbons via hydrogen (AL+HCl) blended with gasoline at maximum torque for motorcycle operation. ARPN Journal of Engineering and Applied Sciences. 10:7780-7783. 2015.
- Flores RM, McDonell VG, Samuelsen GS Impact of Ethane and Propane Variation in Natural Gas on Performance of a Model Gas Turbine Combustor, J. Eng. Gas Turbines Power. 125:701–708. 2003.
- Hassan H and Khandelwal B. Reforming Technologies to Improve the Performance of Combustion Systems, Aerospace 1:67-96. 2014.
- Xiong. H, Wiebenga MH, Carrillo C, Gaudet JR, Pham HN, Kunwar D, et.al. Design considerations for low-temperature hydrocarbon oxidation reactions on Pd based catalysts, Applied Catalysis B: Environmental. 236:436-444. 2018.
- Persson K, Pfefferle LD, Schwartz W, Ersson A, Jaras SG. Stability of palladium-based catalysts during catalytic combustion of methane: The influence of water, Applied Catalysis B: Environmental. 74:242–250. 2007.
- Rubtsov NM, Chernysh VI, Tsvetkov GI, Troshin KYa, Shamshin IO, Kalinin AP The features of hydrogen ignition over Pt and Pd foils at low pressures, Mendeleev Commun. 28:216-218. 2018.
- Rubtsov NM, Chernysh VI, Tsvetkov GI, Troshin KYa, Shamshin IO. Ignition of hydrogen-methane-air mixtures over Pd foil at atmospheric pressure, Mendeleev Commun. 29: (in press). 2019.
- HYPERLINK “http://link.springer.com/search?facet-creator=%22Nickolai+M.+Rubtsov%22″Rubtsov NM. The Modes of Gaseous Combustion. Cham, Springer International Publishing, Switzerland. 2016.
- 13. Markstein GH Cell structure of propane flames burning in tubes, The Journal of Chemical Physics. 17:428-456. 1949.
- Zeldovich Ya B. Theory of Combustion and Detonation in Gases. Acad. Sci. USSR, Moscow, (in Russian). 1944.
- Deutschmann O, Schmidt R, Behrendt F, Warnatz J. Numerical Modeling of Catalytic Ignition, Twenty-Sixth Symposium (International) on Combustion, The Combustion Institute. 1747–1754. 1996.
- 16. Repinski SM. Introduction into chemical physics of the surface of solids. Sibir publishing company, Novosibirsk (in Russian). 1993.
- 17. Semenov N N. On Some Problems of Chemical Kinetics and Reactivity. 2nd edn., Acad.Sci. USSR, Moscow, (in Russian). 1958.