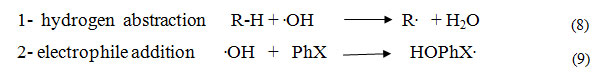Introduction
Thiosemicarbazones and their complexes were the subject of many studies in recent years owing to their potential therapeutic uses, such as antitumoral, fungicidal, bactericidal or antiviral and antiparasitic activities 1-5.Their complexes proved to be good catalysts for a number of organic reactions 6 .They were also used for a range of metal analysis 7, for applications in optical and information instruments and devices 8.
Recently, the photochemical behaviour of some substituted aldehyde thiosemicarbazones was studied. As a result of these studies,it was concluded that the aldehyde residue greatly affects the photoheterocyclization reactions 9. Furthermore, many oxidizing agents were adopted for efficient cyclization of semi-and thiosemicarbazones. In this respect, the regiochemistry of the cyclization reaction to a great extent depends on the conditions of the reaction as well as the structure of the starting compounds 10.
Advanced oxidation process(AOP) that uses hydrogen peroxide with UV- light was found to be an effective method enabling transformation refractory non-biodegradable and/or for toxic organic compounds into only carbon dioxide and water 11-13
Therefore and in order to gain more information about the photochemical behaviour of thiosemicarbazones, the present investigation deals with the photolysis of some thiosemicarbazones of structures ( Fig.1.) and their lead(II) complexes.
2. H2O.](http://unitedjchem.org/wp-content/uploads/2019/02/Vol_2No_1_Cha_Ahm_Fig1-150x150.jpg) |
Experimaental
Materials
All chemicals used were of analytical grades. The ligands used in this study, 2-acetylpyridine- thiosemicarbazone (PTSC), 4-aminoacetophenonethiosemicarbazone(ATSC) and 2-furaldehydethiosemicarbazone (FTSC), were prepared according to published procedures14.
Preparation of Pb(II) complexes
The Pb(II) thiosemicarbazone complexes were prepared by adding an ethanolic solution (20ml) of the thiosemicarbazone ligand (PTSC, ATSC or FTSC) (2 mmol) to an ethanolic solution (10 ml) of the Pb(NO3)2 (1 mmol). The mixture was refluxed on a water bath for 30 min to afford coloured solutions. These solutions were concentrated to half of their volume on a water bath. After cooling to room temperature, the isolated crystalline solids were washed with ethanol and finally dried over CaCl2.
Analysis and Physical Measurements
Carbon, hydrogen, nitrogen and sulfur contents of the complexes were determined by an Elementar-Analysensystem GmbH Vario El analyzer. The i.r. spectra were recorded as KBr pellets on a 470 Shimadzu infrared spectrophotometer. The electronic spectra were measured in DMF solutions on a Perkin Elmer, Lambda 35 UV/VIS spectrophotometer. Electrical conductivity of 10-4 M solution of the complexes were measured at 25 oC on a Jenway 4330 conductivity meter. The thermogravimetric (TG) and differential thermal analysis (DTA) of the solid complexes were preformed using a DTG-60H thermogravimetric analyser (Shimadzu) from ambient temperature to 750 oC with a 10 oC min-1 heating rate in dynamic air atmosphere. The photolyses were achieved using an Osram HBO 200 w/2 Lamp as a light source. Cut-one filter of 299 nm was used. Irradiations were carried out in dimethyl formamide (DMF) solutions in 1-cm spectrophotometer cells at room temperature. Progress of the photolysis was monitored by the above mentioned spectrophotometer.
Results And Discussion
The reaction of lead(II) nitrate with the thiosemicarbazones(L) is illustrated in equation(1).
Pb(NO3)2 + 2L + nH2O (from solvent) → Pb(L)2.nH2O (1)
n = 0, 1 or 4
The resulting complexes are sparingly soluble in common organic solvents but fairly soluble in DMSO and DMF. Table I includes the physical and elemental analysis of these complexes. It should be mentioned that the origin of water included in the formulae of the complexes is the (not absolute) ethanol used for preparation of the complexes.
Conductivity
Conductance measurements of 10-4 M solutions of the complexes in DMF are in the range 132-152 S cm2 mol-1 indicating their 1: 2 electrolytic nature 15.
IR Spectra
Table II includes the IR spectral bands of the complexes. The band around 1600 cm-1 in the spectra of the free ligands (1600 cm-1) is ascribed to to n(C=N) of the azomethine moiety16. Sulphur coordination is manifested by a shift of n(C=S) vibration which appears around 900 cm-1 in the spectra of free ligands17 to lower wave numbers in all the complexes(820-850 cm-1). On the other hand, the bands in the region 3220–3440 cm-1 tentatively ascribed to symmetrical and asymmetrical stretching mode n(NH2) in the spectra of the ligands, undergo a noticeable change in the spectra of the complexes due to coordination of sulfur from the C=S(NH2) group as reported earlier 18. This coordination is confirmed by the presence of a new band at 430–455 cm-1 19, 20 that can be assigned to n (M–N) for all the complexes. The presence of ionic nitrate is indicated by the appearance of a band in the range 1740-1780 cm-1 21.
Electronic spectra
The electronic spectral data of Pb(II) thiosemicarbazone complexes in DMF are recorded in Table III. The highest energy band, observed in the range 37,736-39,524 cm-1, is attributed to a p-p* transition. The band appearing in the spectra of the complexes as a shoulder in the range 27,714-31,446 cm-1 is most probably related to an intraligand charge transfer transition 22.
Thermal studies
The thermal behaviour of the Pb(II) complexes under investigation was studied in dynamic air using thermogravimetric analysis (TGA), derivatives thermogravimety (DTG) and differential thermal analysis (DTA). The thermal decomposition of the complexes was recorded from ambient temperature to 750 0C (Table IV). The results show that the complexes generally decompose in several thermal events, i.e., three or five decomposition steps. The first step for all hydrated complexes represents a dehydration step which correlates well with the theoretical values corresponding to the elimination of water molecules present. The end products of the decomposition were identified from the TG traces to be PbCO3, PbSO4 or PbS.
Based on the above results of elemental analysis, i.r. and electronic spectra, conductivity and thermal analysis, the structure of [Pb(PTSC)2](NO3)2. H2O as an example is proposed and given in Fig. 2.
 |
Photochemical behaviour
Photolyses of the free ligands
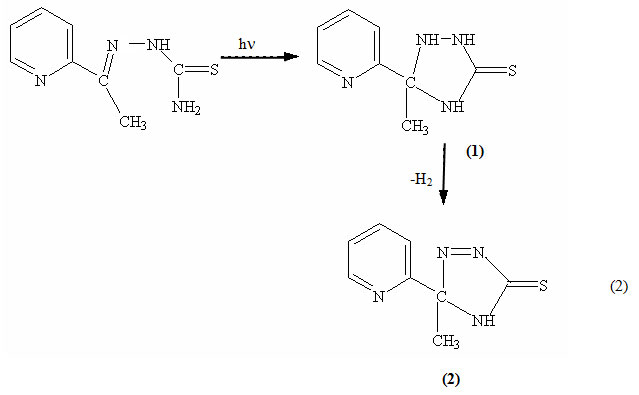
The studied thiosemicarbazones exhibit different behaviour upon irradiating their DMF solutions at λirr ≥ 299 nm and recording their spectral changes at various time intervals. For PTSC, a new band at 444 nm appeared, while the absorbance at 318 nm decreased. The photoreaction is rather clean as indicated by the presence of a sharp isosbestic point at 388 nm (Fig. 4). It is to be noted that the photolysis imparts a yellow color to the solution. For ATSC and FTSC, the spectral changes upon irradiation are manifested by an absorbance decrease at 336 and 360 nm respectively. Such a different photochemical behaviour can be correlated with the variable thiosemicarbazone structures (Fig. 1). The main structure differences are the presence of electron-withdrawing group (pyridyl nucleus) in the PTSC linked to the –C=N-N hydrazone moiety. Based on the photochemical behaviour of some related thioesmicarbazones, a photocyclization reaction could by suggested to account for the development of the new band upon irradiation in case of PTSC. The first step of the photoreaction of PTSC could be the occurrence of a cyclization reaction leading to the formation of 3-methyl-3-pyridyl-1,2,4-triazolidine-5-thione 1, the second step is regarded as a photooxidation of 1 to give 3-methyl-3-pyridyl-∆ 2-1,2,4-triazoline-5-thione 2.In fact the first step takes place only in the case of PTSC and does not occur in case of ATSC and FTSC. It is to be noted that the pyridyl moiety, which acts as electron-withdrawn group may play a crucial role in enhancing the photocyclization reaction. This is not surprising since it is plausible that the electron-withdrawn group attached to C=N-N- in case of PTSC induces a partial positive charge to the carbon atom. At the same time the NH2– group acts as an internal nucleophile, thus driving the cyclization reaction given by equation (2). This result is also confirmed by the observation that there is no cyclization product formed in the acidic medium, since the protonation of the amine group retarded its action greatly as a nucleophilic group so that no cyclization reaction was possible.
Photolyses of Pb(II) complexes
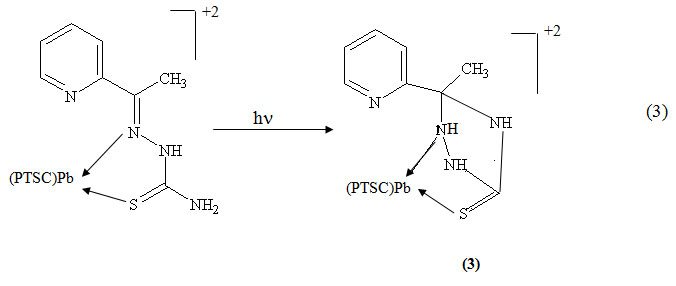
Upon irradiation of the Pb(II) complexes of the thiosemicarbazones under investigation in DMF at λirr ≥ 299 nm, the spectral changes recorded during irradiation show that these complexes behave in a similar mannar to their corresponding free thiosemicarbazones. For the [Pb(PTSC)2](NO3)2. H2O a new band is observed upon irradiation. It is thus proposed that an unstable intermediate 3 forms, which in turn transforms to 2 according to the equation 3:
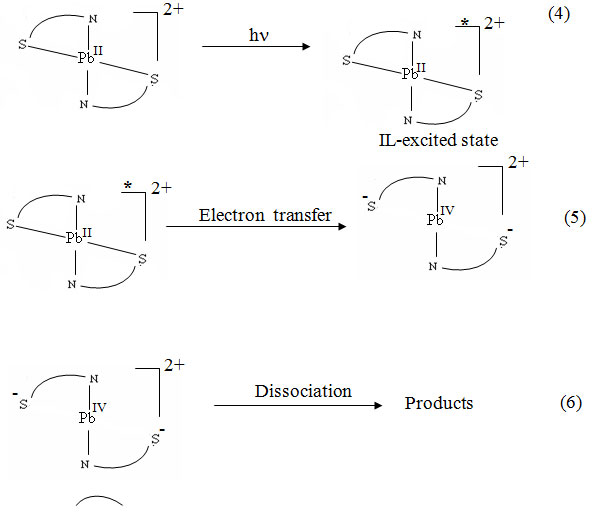
The spectral changes during irradiation (λirr ≥ 299 nm) of DMF solution of both [Pb(ATSC)2](NO3)2 and [Pb(FTSC)2](NO3)2. 4H2O show that the absorbance at 365 nm, assigned to an intraligand transition, decreases. This behaviour indicates the photodecomposition reaction of the complexes. It seems that the active excited state reached by light absorption is the intraligand excited state type. The electronic structure of this excited state formed in the primary photochemical step of course differs from that of the ground state and leads to a change in donor the properties of the ligand 23, which certainly weakens the metal –ligand bond. A reaction scheme given by equations 4-6 is suggested to account for this result.
S N represents a thiosemicarbazone ligand. The IL-excited state generated by light absorption (equation 4) transfers an electron to the S- atom (equation 5). The mechanism of simultaneous decomplexation of two chelate arms (equation 5) is already suggested for the photochemical reactions of some Schiff base complexes 24. The resulting compleces seem to be unstable and decompose to unidentified products in a secondary thermal reaction (equation 6).
It is noteworthy that the similarity of the photochemical behaviour between the free thiosemicarbazones studied and their Pb(II) complexes indicates that the complexation blocked some centers in the ligands which do not participate in the photochemical process namely S and N. These results supported not only the suggested photocyclization mechanism but also the proposed structure of the Pb(II) complexes (Fig. 3).
Photolyses in presence of H2O2
The UV/H2O2 oxidation process is of greatimportance 25, 26. The main feature of such processis the generation of a very powerful oxidizing species, namely hydroxyl radicals 27, 28. The pathway of this process is the reaction of these radicals with saturated aliphatic products that involves abstraction of an H atom in the rate-determing step 29, 30. On the other hand, when the organic molecule contains a double bond, the abstraction of H atom is competed by an addition of .OH radicals on this unsaturated bond 31.
In comparison with other advanced oxidation processes (AOPS), such as fenton, ozone, UV /O3, UV/TiO2, etc. photolysis of hydrogen peroxide shows some advantages such as the complete miscibility of H2O2 with water. Photolysis of hydrogen peroxide is a very simple reaction running with high efficiency:
The hydroxyl radicals generated by H2O2 photolysis in the presence of organic compounds may lead to a variety of reactions, such as:
The radical species formed through the above reactions may cause further reactions leading finally to complete mineralization.
Photolyses in the presence of halocarbon solvents
The radical species formed through the above reactions may cause further reactions leading finally to complete mineralization.
Photolyses in the presence of halocarbon solvents
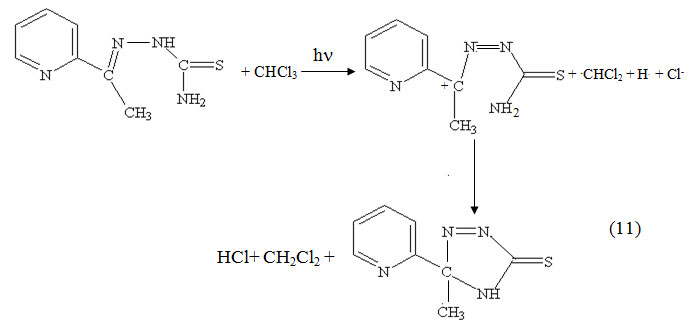
It is known that halocarbon solvents such as CCl4, CHCl3 and CH2Cl2 act as electron acceptors. Therefor, the effect of the presence of 50% of these solvents on the photolysis of thiosemicarbazones and their Pb(II) complexes has been studied(λirr ≥ 299 nm). The results indicated that the photoreaction in presence of such halocarbon solvents are faster than that in pure DMF. To rationalize this behaviourIt it is assumed that an active excited state of the charge transfer to solvent (CTTS) type could be reached by light absorption in addition to the above mentioned intraligand charge transfer excited state. Consequently, the following mechanism is postulated to interpret the formation of photocyclization product in case of PTSC with a higher rate in presence of halocarbon solvents (CHCl3 is taken as an example).It is known that halocarbon solvents such as CCl4, CHCl3 and CH2Cl2 act as electron acceptors. Therefor, the effect of the presence of 50% of these solvents on the photolysis of thiosemicarbazones and their Pb(II) complexes has been studied(λirr ≥ 299 nm). The results indicated that the photoreaction in presence of such halocarbon solvents are faster than that in pure DMF. To rationalize this behaviourIt it is assumed that an active excited state of the charge transfer to solvent (CTTS) type could be reached by light absorption in addition to the above mentioned intraligand charge transfer excited state. Consequently, the following mechanism is postulated to interpret the formation of photocyclization product in case of PTSC with a higher rate in presence of halocarbon solvents (CHCl3 is taken as an example).
Also, The photodissociation increases in the presence of halocarbon solvents. The photodecomposition depends greatly on the nature of the halocarbon solvents and increases in the following order:
CCl4 > CHCl3 > CH2Cl2
This order is in agreement with the redox potential of this halocarbon solvents (CCl4 = – 0.78 V, CHCl3= -1.67 V and CH2Cl2 = – 2.33 V).
Table 1: Physical properties and elemental analysis of the Pb(II) complexes.
| Found /(calc.) | S cm-2 mol– l | m.p/ 0C (Decomp) | Colour | M . F
(M.Wt) |
Complex | |||
| S % | N % | H % | C % | |||||
| 8.39
(8.65) |
19.14
(18.98) |
2.97
3.01)) |
26.14
(26.05) |
152 | 205 | light brown | C16H22N10O7PbS2
(737.80) |
[Pb(PTSC)2](NO3)2.
H2O |
| 8.68
(8.57) |
18.64
(18.73) |
3.37
(3.23) |
28.23
(28.90) |
132 | 216 | Brown | C18H24N10O6PbS2
(747.76) |
[Pb(ATSC)2](NO3)2 |
| 7.91
(8.65) |
14.77
(15.11) |
3.14
(2.99) |
19.39
(19.43) |
143 | 222 | Brown | C12H22N8O12PbS2
(741.68) |
[Pb(FTSC)2](NO3)2. .4H2O |
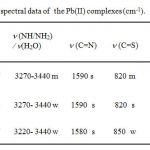 |
Table 3: Electronic spectral data of the Pb(II) complexes (cm-1)
| Assignment | nmax (cm1) | Complex | |
|
IL-charge transfer p- p * transition
|
31,446
37,736 |
[Pb(PTSC)2](NO3)2. H2O |
|
| IL-charge transfer
p- p * transition |
29,718
39,524 |
[Pb(ATSC)2](NO3)2 | |
| IL-charge transfer
p – p * transition |
27,714
38,162 |
[Pb(FTSC)2](NO3)2.4H2O |
 |
References
- H. Beraldo, D. Gambino, Mini Rev. Med. Chem., 4, 31(2004).
- S.Arora, S. Agarwal, S. Singhal, Int. J. of Pharm. and Pharmac. Sci, 6, 34 (2014).
- A. B. M. Ibrahim, M. K. Farh, P. Mayer, Inorg. Chem. Commun., 94, 127 (2018).
- I. R. Barbosa, I.S. Pinheiro,A. D. L. dos Santos, A. Echevarria, C. M. Goulart, G. P. Guedes, N. A. da Costa, B. M. O. e Silva, C. J. Rigar, A. P. Neves, Transition Met. Chem., 43, 739(2018).
- M.Kaushal, S.Indoria, T. S. Lohana, H. Sood, D. S. Arova, G. Hundal, V. A. Smolinski, M. Kaur, J. P. Jasinski, Polyhedron, 148, 9(2018).
- R. Ramachandran, G. Prakash, P. Vijayan, P. Viswanathamurthi, J. G. Malecki, 464, 88(2017).
- H. Nishioka, T. Kumagai, T. Nagahiro, J. Microchem. 50, 88 (1994).
- Y. Tian, C. Duan, C. Zhao, X. You, Inorg. Chem. 36, 1247 (1997).
- L. Limal, D. Grand, V. Vanderesse, R. Marraud, M.. Aubry, Tetrahedron Lett. 35, 3711 (1995).
- S. Buscemi and M. Gruttadauria,Tetrahedron 56, 999 (2000).
- G. M. Colonna, T. Caronna, B. Marcandalli, Dyes and pigments 41, 211(1999).
- C. Galindo and A. Kalt, Dyes and pigments 42, 199(1999).
- M. Muruganadham, M. Swaminathan, Dyes and pigments 62, 269 (2004).
- Z. Yang and R. Yang, Synth. React. Inorg. And Met.Org. Chem., 32, 1059(2002).
- W. J. Geary, Coordination Chem. Rev. (1970).
- A. A. A. Emara and A.A. A. Abou-Hussen, Spectrochim. Acta A, 15(2005).
- L. Lima, L. R. Teixeira, T .G. Carneiro, H. Beraldo, J. Braz. hem. Soc. 10, 184 (1999).
- V. B. Arion, M. A. Jakupec, M. Galanski, P. Unfried and B. K. Keppler, J. Inorg. Biochem. 91, 298 (2002).
- P. Bindu, M. P. Kurup and T. Satyakeerty, Polyhedron 18, 321 (1999).
- L. J. Ackerman, P .E. Franwick, M. A. Green, E. John, W.E. Running, J. K. Swearingen, J.W. Webb and D.X. West, Polyhedron 18, 2759 (1999).
- J. Garcia-Tojal, L. Lezama, J. L. Pizaro, M. Insausti, M.I. Arriortua and T. Rojo, Polyhedron 18, 3703 (1999).
- A. Vogler and H. Kunkely, Coord. Chem. Rev., 251, 577 (2007).
- V. Balazani and V. Carassiti, Photochemistry of Coordination Compounds, Academic Press, London. (1970).
- A H. Osman, Transition Met. Chem. 41, 31(2006).
- S. H. Y, Huang C-R, Chang M-C, Chemosphere 29, 2597(1994).
- C. G. Namboodri, W. K. Walsh, Am. Dyestuff. Reporter 15, (1996).
- J. H. Baxendate, J. A. Wilson, Trans Farady Soc. 344, 53 (1957).
- J. P. Hunt , H. J. Taube, J. Am. Chem. Soc. 74, 5999(1952).
- A. M. Meyerstein, P. Neta, J. Chem. Soc. (1966) 742.
- Y. Ogata Y, K. Tomizawa, K. Takagi. Can. J. Chem. 59, 14(1981).
- D. H. Volmain, J. C. Chen, J. Am. Chem. Soc. 41, 4141(1959).