Introduction
Valacyclovir hydrochloride is L -valine, 2-[(2-amino-1,6-dihydro-6-oxo-9H -purin-9-yl)methoxy] ethyl ester, monohydrochloride [1]. It is an antiviral agent, which is used in the treatment of herpes zoster and herpes simplex virus [1]. Literature review shows minimal methods has been developed and reported for Valacyclovir estimation in biological fluids and there are two methods reported by UV spectroscopy [2-4], HPLC, UPLC and LC-MS methods were reported for estimation of Valacyclovir [5-8]. Method development of HPLC estimation for this Valacyclovir is new method will fulfil all requirements of validation according to ICH guidelines. The increasing importance of speed and reliability of analysis in pharmaceutical analytical laboratories, a new method for determination of valacyclovir in formulations with a short time of analysis is described in this work. It is fast and quick chromatographic method in terms of retention time and run time when compared with other reported methods described in literature survey. The proposed aim of the study was to develop simple, accurate, specific and precise RP-HPLC method for the estimation of Valacyclovir in the bulk and pharmaceutical tablet formulation.
Materials and methods
Chemicals
The Valacyclovir reference standard (RS) was purchased from Sigma, Germany. The Valanext (Valacyclovir 1000mg) tablet marketed drug of Lifestar pharma pvt. Ltd, purchased from vellore, local Pharmacy, India. The HPLC grade acetonitrile, water and methnol was purchased from Sigma, German.
RP-HPLC instrumentation
Shimadzu LC-20 AT HPLC system, using SPD-10 detector (SPD- M20A, Japan). A Zodiac C(18) column (50mm x 4.6mm, 5μm) with Pore size 95Å. The column temperature was maintained at 27ºC and the flow rate was 1ml/min. The injection volume was 20μl, 254nm was set as a wavelength and the HPLC run time was set for 10 minutes.
Preparation of mobile phase
Buffer preparation: Transferred 5 ml of Triethylamine into 1000 ml of water and the PH was adjusted to 3 with Orthophosphoric acid, filtered through 0.45μm nylon membrane filter and degassed. Mobile phase: Buffer and Methanol were mixed in the ratio of 65:35 and sonicated to degas.
Approximately 350 ml of methanol was transferred into 1 liter volumaetric flask and 650 ml of Phosphate buffer was added and mixed thoroughly by shaking and the pH was adjusted to 6 by gradual adding of 0.5N phosphoric acid, the resulting solution was filtered with 0.45μ membrane filter. The final mobile phase was prepared by adding the ratio of 65:35 %. v/v Phosphate buffer: methanol.
Preparation of standard Valacyclovir solution
Accurately weighed 10 mg of pure drug was taken in clean, dry 100 ml volumetric flask and dissolved in small volume of mobile phase and made up the volume to 100 ml with mobile phase. This gave 100 μg/mL of drug concentration. The concentration of 25-125 μg/mL was achived by diluting the standard stock solution with mobile phase.
Pharmaceutical Preparations
Total of 10 tablets were accurately weighed and powdered in a mortar. An amount equivalent to 10 mg of valacyclovir was taken and dissolved in 50 ml of mobile phase and sonicate for 5min. About 50 ml of mobile phase was added and sonicate for further 5 minutes. The mixture was shaking well for 2 minutes and transferred to a 100 ml volumetric flask through a Whatman Filter paper No. 41.The residue was washed thrice with mobile phase and the combined filtrate was made up to the mark with mobile phase, this gave 100 μg/mL of drug concentration. The concentration of 25-125 μg/mL was achived by diluting the standard stock solution with mobile phase. Valacyclovir powder were very freely soluble in water.
Solution stability
The prepared drug solution stability was analysed during the time of analysis and also repeated the same analysis method on same day with different time intervels. The same analysis was repeated after 24hrs by keeping the drug solution under laboratory temperature (37 ± 1°C) and in refrigeration (5 ± 1°C).
Method validation
The present method was preceded to obtain new, sensitive and easy method for simultaneous estimation by HPLC from capsule formulation. According to the ICH guidelines recommendations the experimental was validated and USP-30 for parameters such as, system suitability, accuracy, precision, linearity and specificity.
System suitability
System suitability parameters like resolution, retention time, tailing factor and column theoretical plates was performed by injecting six replicates of standards and two replicates of sample preparation at a 100% level to cross verify the accuracy and precision of the chromatographic system.
Results and Discussion
The RSD values are below 2% indicating the method precision and the accuracy of the method shown by the low standard error values. This shows a good index of accuracy and reproducibility of the developed method. All the parameters including flow rate, detection wavelength sensitivity was maintained constant. The typical RP-HPLC conditions are presented in Table 1.
Table 1: HPLC conditions for estimation of Valacyclovir.
| Parameters | Description |
| Column | Zodiac C(18) column (50mm x 4.6mm, 5μm) |
| Column temperature | 27 ± 1°C |
| Mobile phase | Phosphate buffer: methanol (65:35 %. v/v) |
| Detection | Photodiode array detection at 230 nm |
| Injection volume | 20 μl |
| Flow rate | 1 ml min-1 |
The HPLC chromatogram of Valacyclovir standard and Valacyclovir is presented in figure 1 and 2.
 |
 |
Figure 2: A Typical Chromatogram of Valacyclovir tablet formulation |
Linearity
The proposed method linearity was examined for five different concentrations. The concentration ranges from 25-125 μg/ml. The valacyclovir standard linearity was determined by the plotting graph concentration vs peak area. By peak area as a functional of analyte concentration linearity was evaluated for valacyclovir. The linearity graphs presented in figure 3, and data presented in Table 2. The system suitability is demonstrated by the linearity analysis.
Table 2: HPLC linearity data for Valacyclovir
| Concentration (µg/ml) | Peak area |
| 25 | 254567.67 |
| 50 | 502344.22 |
| 75 | 754352.35 |
| 100 | 1006097.45 |
| 125 | 1247649.79 |
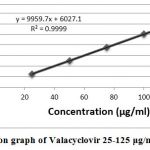 |
Figure 3: Calibration graph of Valacyclovir 25-125 μg/ml precision |
Accuracy
The recovery experiment showed the accuracy of the method. The good recovery showed the method was accurate. The analysis for recovery was performed by known amount of valacyclovir working standard added to pre-analyzed solution of the formulation in the test concentration range of (40%, 60% and 100 %). For each recovery level three samples was prepared and repeated for 3 consecutive days. The statistical results for recovery study are well within the range (S.D. < 2.0). The valacyclovir tablet formulation recoveries results are presented in Table 3.
Table 3: Recovery studies of Valacyclovir tablet formulation.
| Drug | % Level | Amount Added
(mg) |
Amount Found
(mg) |
% Recovery | Mean recovery |
| Valacyclovir | 50 | 50 | 100 | 99.77 |
99.59
|
| 50 | 100 | 149 | 99.45 | ||
| 50 | 150 | 199 | 99.55 |
Precision
The proposed method precision (repeatability) experiment results of are shown in Table 4. In the proposed method intraday and interday precision was examined by analyzing the responeses of the sample on the same day for 4 repeatations and 3 alternate days for 2-10 μg/ml concentration range of valacyclovir. The obtained results are represented in % RSD. The % CV of the proposed method was precise as the values < 1.0 % for the repeatability study. The precision data are presented in Table 5.
Table 4: Method precision data of Valacyclovir by RP-HPLC method
| Valacyclovir 50μg/ml (n=4) | Retention time | Area |
| 1 | 2.101 | 501523.22 |
| 2 | 2.091 | 501193.20 |
| 3 | 2.107 | 499634.79 |
| 4 | 2.103 | 500553.52 |
| Mean | 3.10 | 507751.18 |
| S.D a | 0.0201 | 101.71 |
| % CVb | 0.68 | 2.07 |
n=4 observations
Table 5: Intermediate precision data of Valacyclovir by RP-HPLC method
| Valacyclovir μg/ml | Inter-day measured mean area ± S.D.a | %CVb (nc=4) | Intra-day measured mean area ± S.D.a | %CVb (nc=4) |
| 75 | 4057.25± 2.19 | 0.0872 | 4497.44± 4.65 | 0.0125 |
| 100 | 7919.75±2.22 | 0.0617 | 7172.75±2.15 | 0.0157 |
| 125 | 99613.12±2.11 | 0.0722 | 9576.12±4.26 | 0.1056 |
nc = 4 observations
Limit of detection and quantitation:
The limit of detection and quantification for valacyclovir is presented in table 6. Limit of detection (LOD) and limit of quantification (LOQ): LOD and LOQ were estimated by injecting the diluted known concentration solution, which gave minimum detectable peak area. This was multiplied thrice to get LOD and by 10 times to get LOQ with suitable precession as per the International Conference on Harmonization guidelines. LOD and LOQ were calculated which was found at concentrations of 0. 55μg/mL and 1.05 μg/mL respectively.
Table 6: Results of Limit of detection & limit of quantification
| Parameters | Valacyclovir |
| LOD (µg/ml) | 0.55
|
| LOQ (µg/ml)
|
1.05 |
Specificity
Specificity of chromatographic method was established by the separation of drug peak from the adjacent resolving peaks. Specificity was verified by running a blank, placebo and drug working solution to check any interference at the retention time of the standard peak. The valacyclovir standard reference and the drug formulation show the specificity of the method. The RP-HPLC chromatogram of valacyclovir both bulk and the tablet formulation are presented in figure 1, 2. The valacyclovir standard reference and tablet formulation retention time was found to be 2.10 min. For the tablet formulation there was no excipient interference was detected, which shows the specificity of the method. The proposed method showed the ability to determine the analyte in presence of excipients.
The system suitability
For the system suitability parameters five repeats of standards and two repeats of sample preparation are injected, the data is presented in table 7. The Assay data of valacyclovir presented in table 8.
Table 7: Results of system suitability parameters
| SNo | Parameters
|
Valacyclovir |
| 1. | Theoretical plates | 4790 |
| 2. | Tailing factor | 0.968 |
| 3. | Resolution factor | 2.11 |
| 4. | Retention time | 2.1± 0.1 |
| 5. | Calibration range or Linear dynamic range | 25-125μg/ml |
Table 8: Quantitative estimation (Assay) data of Valacyclovir
| Drug
|
Label claim (mg) | Amount found
mg) |
Mean amount found
(mg/ ml) |
Percentage purity
(% w/w) |
Mean purity (% w/w) | % Deviation |
|
Valacyclovir |
50 |
50.01
49.77 50.21 49.93 50.14 |
50.01 |
100.17
99.49 100.21 99.77 100.14 |
99.95 |
+ 0.2
+0.1 +0.2 +1.0 +0.4 |
n= 4 observations
Statistical Parameters
The results of assay obtained are subjected to the following statistical analysis, standard deviation, relative standard deviation, coefficient of variation and standard error. are presented in table 9.
Table 9: Results of statistical parameters Statistical parameters
| SNo | Parameters | Valacyclovir |
| 1. | Standard deviation (SD) | 1.17 |
| 2. | Relative standard deviation (RSD) | 0.0576 |
| 3. | % RSD | 0.576 |
| 4. | Standard error (SE) | 0.02077 |
| 5. | Correlation Coefficient (r) | 0.9999 |
| 6. | Slope (a) | 9959.7 |
| 7. | Intercept (b) | 6027.1 |
Conclusion
The proposed and developed RP-HPLC method is precise, accurate, and sensitive. The method is rapid, reproducible, and economical and does not have any interference due to the excipients in the pharmaceutical preparations.
Acknowledgement
The author is thankful and acknowledges Jazan University for required facilities to carry out this research work.
References
- United States Pharmacopeia, National Formulary-29, Rockville, USA: United States Pharmacopeial convention; 2011.
- Aswani Kumar, CH.; Anil Kumar, T.; Gurupadayya, B.M.; Sloka, S.N.; Rahul Reddy, M.B.; Novel spectrophotometric determination of valacyclovir and cefotaximeusing 1, 2- napthaquinone-4-sulfonic acid sodium in bulk and pharmaceutical dosage form, Archives of Applied Science Research. 2010, 2(4): 278-287.
- Reddy, J.S.; Maqsood Ahmed, M.S.; Chakravarthi, I.E.; Prabhavathi, K.; Spectrophotometric estimation of valacyclovir in pharmaceutical preparations. J Chem Pharm Res 2011, 3(7): 773–776.
- Srihari, G(2) Reddy N.U.M.; Chakravarthi, I.E.;A simple spectrophotometric determination of valacyclovir in pharmaceutical preparations. J Curr Chem Pharm Sci, 2011, 1(7): 15–18.
- Rasool, S.K.; Naik, D.V.; Prasad Babu, D.; Buchi, N.; RP-HPLC method for the estimation of valacyclovir in bulk and pharmaceutical formulations, International Journal of Pharmacy and Pharmaceutical Sciences. 2012, 4(1): 214 -218.
- Sugumaran, M.; Bharathi, V.; Hemachander, R.; Lakshmi, M.; RP- HPLC method for the determination of Valacyclovir in bulk and Pharmaceutical formulation, Der Pharma Chemica. 2011, 3(4): 190-194.
- Sasanya, J.J.; Abd-Alla, A.M.; Parker, A.G.; Cannavan, A.; Analysis of the antiviral drugs acyclovir and valacyclovir- hydrochloride in tsetse flies (Glossina pallidipes) using LC-MS, Journal of Chromatography B- Analytical Technologies in the Biomedical and Life Sciences, 2010, 878(26): 2384- 2390.
- Patil, G.; Yeole, P.G.; Wadher, S.J.; A validated specific reverse phase liquid chromatographic method for the determination of valacyclovir in the presence of its degradation products in bulk drug and in tablet dosage forms. Int J ChemTech Res, 2009, 1(2): 16–26.
