Introduction
One of such potentially active heterocyclic moiety is triazine moiety.1-2 During last few years the potential of s-triazine derivatives have found extensive applications in molecular recognition, agrochemical, pharmaceutical, textile, plastic, rubber industries and also used as pesticides, dyestuffs, optical bleaches, explosives, surface active agents and medicinal properties has been investigated to larger extent.3-29 The s-triazine play a significant role not only in life science but also in many other industrial fields related to unique and superior chemistry. Among them 1, 3, 5-triazines represent a widely used lead structure with interesting applications in various fields.
The information available in the literature has been used as a guideline for the synthesis of various triazine based heterocyclic compounds and study their biological activity. The synthesis of the prominent triazine related heterocyclic compounds revealed through the literature study are discussed below.
Geoffrey et al.30 have been reported the triamino triazine DNA helicase inhibitor with biological activity and tested a chemical library in a DNA helicase asssay with good activity but associated cytotoxicity towards mammalian cells.
Sunduru et al.31 reported a synthesis and characterization of 2, 4, 6-trisubstituted triazines and shown anti-malarial activity against P. falciparum. A variety of compound synthesized which shows MIC in range of 1-2 µg/ml and shows anti-malarial activity several times more than cycoguanil.
Mingfang et al.32 reported the synthesis and anti-tumor activity of new triamino triazine derivatives and screened them for inhibition activities to colorectal cancer (CRC) cell lines (HCT-116, HT-29). Many of synthesized compounds exhibit anti-proliferatory effect on both HCT-116 and HT-29 cell lines at 10 µМ concentration.
Antan et al.33 reported the synthesis and characterization of regioisomeric 5(7)-amino-6,7(4,5)dihydro[1,2,4]triazolo[1,5-a][1,3,5]triazines. The biological activity of potent agent pyrazoline, aminopyrimidine and isooxazoline are studied.
Yuan et al.34 have been reported the synthesis of new series of diaryl triazine and diaryl pyrimidines which have extremely potent nucleoside reverse transcriptase inhibitor action with microbicides and also shows antiviral and immune suppressive activity of new classes of non-nucleoside reverse transcriptase inhibitors.
Menicagli et al.35 reported in solid-liquid phase transfer condition, both primary and secondary alcohols react cleanly with 2,4,6-trichloro-1,3,5-triazine to afford the mono or dialkoxy derivative depending on the reagent molar ratio.
 |
Mc Namara et al.36 reported the novel synthesis of Sharpless asymmetric ligand using cyanuric chloride, 4-bromoaniline and quinine. The new ligand catalyst give good yield in dihydroxylation of alkene.
Yuan et al.37 reported the synthesis and characterization of diaryl triazine with effective anti-HIV activity.
Chang et al.38 reported a new orthogonal method for solid phase synthesis of 2, 4, 6-triazine. They joined aldehyde resin to primary amine by reductive amination. This was reacted with mono substituted dichloro triazine. The trisubstitued derivative obtained by nucleophilic reaction with amine.
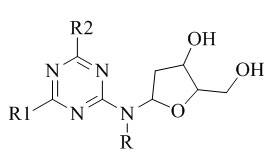
Mulwad et al.39 reported the synthesis, characterization and evaluated the stability of triazine nucleosides.
Kuplich et al.40 previously studied the synthesis of novel fluorescent triazine derivative which obtained from nucleophilic aromatic substitution of CC. Fluorescent cyanuric derivative reacted with cellulose fibers to give novel fluorescent celluloric material.
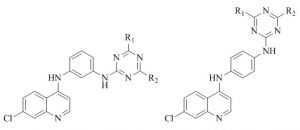
Kumar et al.41 further developed the synthesis of 4-anilinoquinoline triazines derivatives and tested them for their antimalarial activity against CQ-sensitive 3D7 strain of P. falciparum as well as for their cytotoxicity toward VERO cell line. Five compounds exhibited the antimalarial potency superior to CQ. A few compounds were found to be orally active at a dose of 100 mg/kg and check it for 4 days against CQ-resistant strain of P. yoelii. The inhibition of b-hematin formation assay and molecular docking study has been conducted in order to gain insight into the mechanism of action of proposed targets for the 4-anilinoquinoline and triazine moiety of the hybrid compounds.
Simanek et al.43 reported a complex dendrimers with varied peripheries as proof-of-concept, less complex molecules tailored for specific applications including DNA and RNA delivery and drug decorated dendrimers for potential therapeutic applications including infectious disease and cancer. These syntheses have been executed at scales that range from high milligrams to over a kilogram. The nucleus of early dendrimers was a simple diamine, including piperazine, yielding the so-called bow-tie structures, middle period targets boast either a trispiperazinyl triazine center or a ‘super-core’ with six piperazine groups. The p-amino benzylamine was replaced by piperazine and then by aminomethyl piperidine with more exotic diamines sprinkled in throughout.
Gupta et al.44 reported the synthesis and bioevalution of 4-aminoquinoline triazine as a antimalarial agent. A series of new hybrid 4-aminoquinoline triazine were synthesized and screened against CQ strain 3D7 of P. falciparum in vitro.
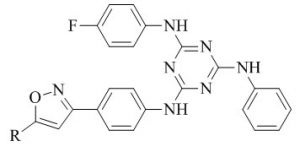
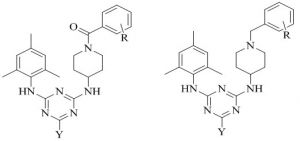
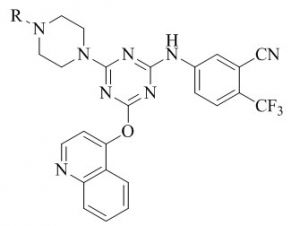
Xuwang Chen & co‐authers45,46identified a novel series of triazine substituted piperidine derivatives and screened for anti‐HIV activities in MT‐4 cells. Many hybrids have exhibited potent activity against wild type HIV‐1 with EC-50 values in little nanomolar concentration than that of standereds Delavirdine, Dideoxycitidine, Zidovudine and Nevirapine.
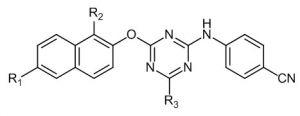
Where R = -OMe , -NO2, -CN , -COOH , -F. Y = OMe , -NH2 , -NHMe.
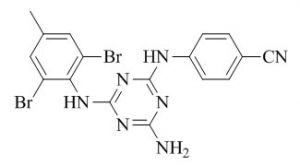
More recently, Desai et al.47 have synthesized a variety of N-substituted piperazine annulated s-triazine derivatives and performed bioevaluation of a sequence of 2,4,6-trichloro-1,3,5-s-triazine analogues which on substitution with anisole, 4-hydroxy coumarin and different N-substituted piperazine derivatives on the C-6 position of s-triazine nucleus. The resultant compounds were screened for their in vitro antimicrobial activity against gram negative bacteria (E. coli, K. pneumoniae), gram-positive bacteria (S. aureus, B. subtilis) and fungal species (C. albicans and S. cerevisiae) using the disc diffusion method. Most of the synthesized compounds were appeared with promising antimicrobial activity.
Shen et al.48 studied and reported a various triazine derivatives as ROCK1 (Rho‐associated protein kinases) inhibitors by with a computational protocol that combines molecular docking, binding free energy calculations, molecular dynamics (MD), simulations and binding energy decomposition analysis. The some of them showed promising potency.
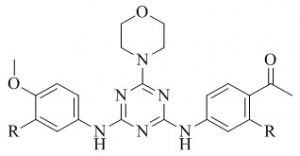
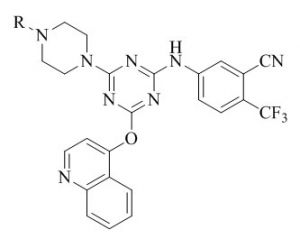
Patel et al.49 have previously studied facile synthesis of benzonitrile/nicotonitrile based s-triazines as new potential antimycobacterial agents. A general strategy to synthesize 4/ 6-(4-(4-methylpiperazin-1-yl)-6-(4-(4-oxo-2-phenylthiazolidin-3-yl) phenyl)-1, 3, 5-triazin-2-yloxy) benzonitriles / nicotinonitriles was developed by applying an efficient palladium-catalyzed CeC Suzuki coupling and synthesized compounds were characterized by spectral analysis.
 |
Lee et al.50 reported a series of chalcone–triazine fusion library has been developed. A planned fused chalcone–triazine structure by combining a fluorophore and a biophore for imaging and biological probe growth. By using solid support Chemistry, 80 analogs with different amine building blocks were prepared. Spectroscopic studies and cell image screening of the hybrid compounds indicate their potential to be used as probes for bio-imaging.
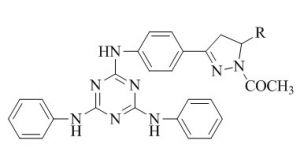
Solankee et al.51 have developed the synthesis, characterisation and bioevalution of novel fluorine containing s-triazine based chalcone and the synthesized compound screen for their anticancer and antibacterial activity.(Scheme 3).
 |
Solankee et al.52 was previously studied the synthesis of various new s-triazine based Chalcone as potent antimicrobial agent using cyanuric chloride. s-triazine chalcone act a improved bioactivity as local anaesthetic, antibacterial, antimalerial, antiprotozoal, antitubercular, anticancer and antifungal agents.(scheme 4)
 |
Solankee et al.53 reported a synthesis and characterization of chalcone as antibacterial agents. The compounds have been screened for antibacterial activity against E. coli, S. Paratyphil-A, S. aureus and B. subtilis. (scheme 5)
 |
Solankee et al.54 was have accomplished synthesis and characterization of some novel chalcones and their derivatives bearing s-triazine core.(scheme 6)
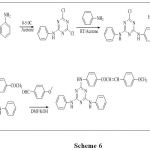 |
Solankee et al.55 reported synthesis of various new series s-triazine chalcones. The characterization of newly synthesized compounds has been done on the basis of IR, 1H NMR and mass spectral data as well as elemental analysis.(scheme 7)
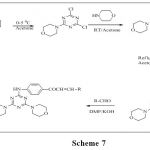 |
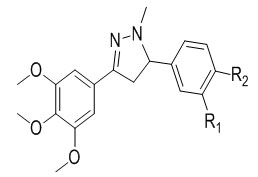
Solankee et al.56 reported synthesis of various new series triazine Chalcones which on cyclisation with hydrazine hydrate in presence of acetic acid give triazine based acetyl pyrazolines. The characterization of newly synthesised compounds has been done on the basis of IR, 1H NMR and mass spectral data as well as elemental analysis.
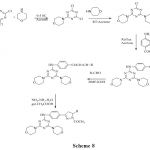 |
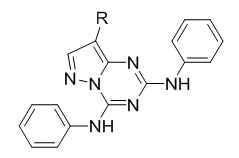
Zhe et al.57 designed, synthesis, and anticancer activity of new inhibitors of protein kinase CK2 . Using pyrazolo[1,5-a][1,3,5]triazine as the core scaffold, a structure-guided chain of modifications provided pM inhibitors with lM-level cytotoxic activity in cell-based assays with prostate and colon cancer cell lines.
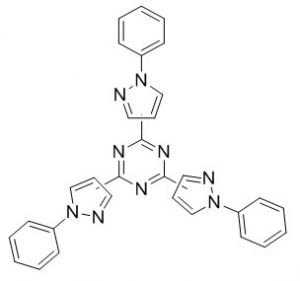
Anatonio et al.58 synthesis of various new tris-pyrazolyl-1,3,5-triazines have been prepared by cyclotrimerization of aromatic nitriles in solvent free conditions. The resulting compounds are used for application in crystal engineering and coordination chemistry.
Solankee et al.59 reported synthesis of various new s-triazine based Chalcone and their pyrazoline derivative as potent antimicrobial agent using cyanuric chloride.
Bonesi et al.60 have accomplished a series of pyrazole were prepared to investigate potentially activity as angiotensin I-converting enzyme (ACE) inhibitors. The structure of compounds were verified by elemental analysis, IR, 1HNMR, and mass experiments.
Palaska et al.61 have synthesized a series of 1,3,5-triphenyl-2-pyrazolines and these compounds showed significant antidepressant activity when compared to the standard antidepressant drugs clomipramine and tranylcypromine
Solankee et al.62 reported synthesis and characterization of chalcone, pyrazoline as antibacterial agents. The compounds have been screened for antibacterial activity against E. coli, S. paratyphil-A, S. aureus and B. subtilis.
 |
Conclusion
The synthesis of the triazine derivatives are illustrated in this review will be useful for further development of medicinal chemistry and applications of new triazine-based heterocyclic promising building block. These findings encourage the scientific community towards the optimization of the pharmacological profile of this structural moiety as an important scaffold for the treatment of oxidant related diseases. To further optimize the complete potential of triazine compounds, the SAR-based study will likely continue to play an important role and further investment in developing fundamental genetic systems. This information may endow with an opportunity to scientific community to design selective, optimized as well as poly-functional triazine analogs for the treatment of multifactorial diseases.
Conflict of interest
The authors confirm that this article content has no conflicts of interest.
Acknowledgements
The authors’ thanks and greatly acknowledge the Principal of Dayanand science College, Latur, Maharashtra and HOD of Chemistry Department Dr. Y. P. Sarnikar for providing laboratory facility and consistent support.
References
- Quirke, M. E.; Katritzky, A. R.; Rees, C. W. ; Pergamon: New York, N.Y, 1984, 3, 457–530.
- Giacomelli, G.; Porcheddu, A.; De Luca, L. Org. Chem. 2004, 8(15), 1497–1519.
- Smolin, E. M.; Rapoport, L. s-Triazines and Derivatives. Interscience Publishers, Inc.: New York, 1959, 13, 644.
- Leach, M. J.; Baxter, M. G.; Critchley, M. A. E. Epilepsia. 1991, 32(2), S4.
- Blaney, J. M.; Hansch, C.; Silipo, C.; Vittoria, A. Rev. 1984, 84, 333-407.
- Quirke, J. M. E. Comprehensive Heterocyclic Chemistry. Pergamon Press: Oxford, 1984, 3, Pt. 2B, 457.
- Eric, E.; Hanan, A.; Sanjiv,L.; Jongdoo, L.; Meredith, M.; Vincent, J. V.; Brandon, V. Royal Soc. A. 2010, 466, 1445-1465.
- Blotny, G. Tetrahedron 2006, 2, 9507–9522.
- Mur, V. I. Chem. Rev. 1964, 33, 92–103.
- Menicagli, R.; Malanga, C.; Peluso, P. Commun. 1994, 24, 2153-2158.
- Samaritani, S.; Peluso, P.; Malanga, C.; Menicagli, R. J. Org. Chem. 2002, 1551-1555.
- Gröger, H.; Sans, J.; Güthner, T. Oggi. 2000, 18, (3/4).
- Huthmacher, K.; Most, D. In Ullmann’s Encyclopedia of Industrial Chemistry, 7th ed.; Wiley-VCH: Weinheim, Germany,
- Kaminski, Z. J.; Kolesinska, B.; Kaminska, J. E.; Gora, J. Org. Chem. 2001, 66, 6276-6281.
- Gröger, H.; Sans, J.; Güthner, T. Oggi. 2000, 18(3/4), 12-16.
- Knuesli, E. Residue Rev. 1970, 32, 1-9.
- Avupati, V. R.; Yejella, R. P.; Parala, V. R.; Killari, K. N.; Papasani, V. M. R.; Cheepurupalli, P.; Gavalapu, V. R.; Boddeda, B. Med. Chem. Lett. 2013, 23, 5968-5970.
- Bhat, H. R.; Singh, U. P.; Gahtori, P.; Ghosh, S. K.; Gogoi, K.; Prakashe, A.; Singh, R. K. New J. Chem. 2013, 37, 2654-2662.
- Taranalli, A. D.; Bhat, A. R.; Srinivas, S.; Saravanan, E. J. Pharmaceutical Sci. 2008, 70, 159-164.
- Karali, N.; Gürsoy, A.; Kandemirli, F.; Shvets, N.; Kaynak, F. B.; Ozbey, S.; Kovalishyn, V.; Dimoglo, A. Future Med. Chem. 2007, 15, 5888-5904.
- Kaminskyy, D.; Bednarczyk-Cwynar, B.; Vasylenko, O.; Kazakova, O.; Zimenkovsky, B.; Zaprutko, L.; Lesyk, R. Chem. Res. 2012, 21, 3568-3580.
- Wang, S.; Zhao, Y.; Zhu, W.; Liu, Y.; Guo, K.; Gong, P. Archiv der Pharmazie 2012, 345, 73-80.
- Havrylyuk, D.; Zimenkovsky, B.; Vasylenko, O.; Gzella, A.; Lesyk, R. Med. Chem. 2012, 55, 8630-8641.
- Balzarini, J.; Orzeszko-Krzesi_nska, B.; Maurin, J. K.; Orzeszko, A. J. Med. Chem. 2009, 44, 303-311.
- Palekar, V. S.; Damle, A. J.; Shukla, S. R. J. Med. Chem. 2009, 44, 5112-5116.
- Omar, K.; Geronikaki, A.; Zoumpoulakis, P.; Camoutsis, C.; Sokovi, M.; Ciri, A.; Glamoclija, J. Future Med. Chem. 2010, 18, 426-432.
- Shih, M. H.; Ke, F. Y. Future Med. Chem. 2004, 12, 4633-4643.(d) Terzio_glu, N.; Karali, N.; Gürsoy, A.; Pannecouque, C.; Leysen, P.; Paeshuyse, J.; Neyts, J.; De Clercq, E. Arkivoc (Archive for Organic Chemistry) 2006, 1, 109-118.
- Ragab, F. A.; Eid, N. M.; el-Tawab, H. A. Pharmazie 1997, 52, 926-929.
- Raikwar, D.; Srivastava, S. K.; Srivastava, S. D. Ind. Chem. Soc. 2008, 85, 78-84.
- Geoffrey, A. M.; Rang Reddy, F.; Arhin, A.; Belley, D.; Lehoux, Greg M. I. ; Sarmiento, T. R.; Parr , P.; Gross, J.; Pelletier, A.; Rafai , F. Med. Chem. 2006, 16, 1286-1290.
- Sunduru, N.; Agarawal, A.; Sanjay, B. K. Med. Chem. 2006, 14, 7706 – 7715.
- Mingfang, Z.; Chenghui, X.; Jianwei, M.; Yan. J. Med. Chem. 2007, 15, 1815-1827.
- Anton, V. D.; Anna, V. D., Tetrahedron 2007, 63, 12888-12895.
- Yuan, Z. X.; Chen, F. E.; Jan, B. E.; Clercq, D., J. Med. Chem. 2008, 43, 1230-1236.
- Menicagli, R.; Malanga, C.; Peluso, P., Commun.1994, 24(5), 2153-2158.
- Mc Namara, C. A.; King, F.; Bradley, M., Tetrahydron Lett. 2004, 26–27.
- Xiong, Y. Z.; Che, F. E.; Jan, B.; Clercq, E. D., J. Med. Chem. 2008, 43, 1230–1236.
- Moon, H. S.; Jacobson, E. M.; Khersonsky, S. M.; Luzung, M. R.; Walsh, D. P.; Xiong, W.; Lee, J. W.; Parikh, P. B.; Lam, J. C.; Karg, T. W.; Rusania, G. P.; Schier, A. F.; Chang, Y. T. Am. Chem. Soc. 2002, 124, 11608-11609.
- Mulwad, V. V. Indian J. Chem. 2003, 42(B), 2583-2588.(b) Ronchi, S.; Prosperi, D.; Compostella, F.; Panza, L. Synlett 2004, 1007–1010.
- Kuplich, M. D.; Grasel, F. S.; Campo, L. F.; Rodembuch, F. S.; Stefani, V. S., Brazilian Chem. Soc. 2011, 1-7.
- Kumar, A.; Srivastava, K.; Kumar, R. S.; Siddiqi, M. I.; Puri, S. K.; Sexana, J. K.; Chauhan, Prem M. S. J. Med. Chem. 2011, 46, 676-690.
- Patel, R. V.; Kumari, P.; Rajani, D. P.; Chikhalia, K. H. Eur. J. Chem. 2011, 46, 4354-4365.
- Simanek, E. E.; Abdou, H.; Lalwani, S.; Lim, J.; Mintzer, M.; Venditto ,V. J.; Vittur, B. Proc. R. Soc. A 2010, 466, 1445–1468.
- Gupta, L.; Pal, P.; Khan, S. Eur. J. Med. Chem. 2010, 45, 2265-2276.
- Chen, X.; Zhan, P.; Liu, X.; Cheng, Z.; Meng, C.; Shao, S.; Pannecouque, C.; Clercq, E. D.; Liu, X. Bioorg. Med. Chem. 2012, 20(12), 3856‐3864.
- Chen, X.; Zhan, P.; Pannecouque, C.; Balzarini, J.; De Clercq, E.; Liu, X. Eur. J. Med. Chem. 2012, 51, 60‐66.
- Desai, S. D.; Mehta, A. G. Research J. Chem. Sci. 2014, 4(5), 19-14.
- Shen, M.; Zhou, S.; Li, Y.; Pan, P.; Zhang, L.; Hou, T., BioSyst. 2013, 9 (3), 361‐374.
- Patel, A. B.; Chikhalia, K. H.; Kumari, P., J. Med. Chem. 2014, 79, 57-65.
- Lee, S. C.; Zhai, D.; Chang, Y. T. Tetrahedron Lett. 2013, 54, 2976–2979.
- Solankee, A.; Prajapati, Y. Rasayan J. Chem. 2009, 2(1), 9-14.
- Solankee, A.; Kapadia, K.; Ciric, A.; Sokovic, M.; Doytchinovo, I.; Geronikaki, A. Eur. J. Med. Chem. 2010, 45, 510–518.
- Solankee, A.; Lad, S.; Solankee S.; Patel, G. Ind. J. Chem. 2009, 48(B), 1442-1446.
- Solankee, A.; Kapadia, K.; Patel, H.; Solankee, P. & S.; Prajapati, Y. Ind. J. Chem. 2008, 47(B), 473-476.
- Solankee, A.; Patel, R.; Patel, K. Der Pharma Chemica. 2011, 3(6), 317-324.
- Solankee, A.; Patel, R.; Patel, K. Der Pharma Chemica, 2011, 3(6), 317-324.
- Zhe, N.; Carin, P.; Philip, E.; Stephen, M.; Robert, A.; Jia, Lu.; April. A.; Kraig, M. Y.; Shaosong, C. Med. Chem. Lett. 2007, 17 4191–4195.
- (a) Antonio, H.; Angle, D. O.; Jose, E.; luis, J. M.; Andres, M.; Ana, S. M. Tetrahydron 2001, 57,4397-4403.(b) Bansal, E.; Srivastava, V. K.; Kumar, A. J. Med. Chem. 2001, 36, 81.
- Solankee, A.; Kapadia, K.; Ciric, A.; Sokovic, M.; Doytchinovo, I.; Geronikaki, A. J. Med. Chem. 2010, 45, 510.
- Bonesi, M.; Loizzo, M. R.; Statti, G. A.; Michel, S.; Tillequin, F. Med. Chem. Lett. 2010, 20, 1990.
- Palaska, E.; Erol, D.; Demirdamar, R. B. J. Med. Chem. 1996, 31, 43.
- Solankee, A.; Lad, S.; Solankee, S.; Patel, G. Ind. J. Chem. 2009, 48(B), 1442-1446.