Introduction
Rose Bengal [IUPAC name 4,5,6,7-Tetrachloro-3,6’-dihydroxy-2,4,5,7-Tetraiodospiro (isobenzofuran-1 (3H), 9-(9H) xanthen)-3-one] is popularly known as Acid Red 94, Food Red Dye 105, Bengal Rose or Red No.105 (Fig. 1). It is a well known fluorescein class of halogen containing highly water soluble, anionic dye. It is widely used in liver function test and in the eyes to stain necrotic tissue and devitalized cells of the cornea in conjunctivitis1. The toxicity of Rose Bengal is mainly due to presence of halogen atoms in it Sako et al.2, 3. Tabery H.M., Lee et al.4, 5 reported that the higher concentration of the Rose Bengal dye poses toxic effects on human corneal epithelium cells and damage eyes. Danylkova et al. 6 investigated toxic effects of Rose Bengal on the optic nerves of humans and long and short term exposures of the dye was found to causes severe optic nerve injury leading to permanent vision losses. The dye also affects brain cells of humans and animals. Kelly et al. and Levitan et al. 7,8 investigated the effect of Rose Bengal on tissue cells of brain and prothrombosis brain infarction. The dye is extremely hazardous when comes in contact with skin and creates irritation, itching, scaling, reddening and even blistering. On ingestion by inhalation it damages mucous membranes, which develops respiratory irritations in humans9. Hence, it is considered worthwhile to develop detailed procedures for the removal and recovery of the Rose Bengal dye through adsorption over waste material – De–Oiled Soya a waste product.
The attempts made in the past using adsorption technique are also not on easily available waste materials. Therefore use of De–Oiled Soya, well known waste materials, as adsorbent is an innovative and thoughtful attempt to economically and safely eradicate the hazardous dye Rose Bengal. The present research describes a detailed column study on the removal and recovery of Rose Bengal dye from the wastewaters using a De–Oiled Soya.
 |
Material and Methods
The Rose Bengal dye is a red color and water soluble powder having molecular formula C20H2Cl4I4Na2O5 (molecular weight 1017.65). It is obtained from M/S Merck and used as procured. A commonly known synonyms for Rose Bengal is halogen containing dyes. Double distilled water was used for the preparation of 0.001 M stock solution of the Rose Bengal dye. De–Oiled Soya is a byproduct of soyabean oil industries, which is obtained after extracting almost all nutrient and proteins from the soyabean procured from M/s Surya Agro Oils, Bhopal, India. All other reagents used in this investigation were of A. R. grade.
The pH of each solution was measured by using microprocessor based pH meter model number HI 8424 (M/s Henna Instruments, Italy). Spectrophotometric experiments were carried out in the wavelength range 200 – 700 nm using UV/Visible spectrophotometer, Model 117 (Systronics, Ahmedabad, India). Scanning electron microscopy was performed using a Philips SEM 501 electron microscope. Philips X-ray diffractophotometer employing nickel filtered Cu-α- radiations were used for x-ray measurements. Mercury porosimeter was used to determine the porosity, specific gravity bottles were employed to find out density and Quantasorb Model QS-7 surface area analyzer was used to measure surface area of De–Oiled Soya granules.
Experimental
Material development
De–Oiled Soya was ground into very small granules, and then treated with hydrogen peroxide solution (30% w/v) for 24 h at room temperature followed by washing. It was then kept into the electric oven at 100 0C for about 1 h to remove all possible volatile matters from adsorbent. Finally the adsorbent was sieved to various mesh size and stored in separate vacuum desiccators until needed.
Column Adsorption Study and Regeneration
To evaluate the practical utility of the adsorbents, bulk adsorption was carried out by column operations. The column studies were carried out by using a long tubular glass column of 30 cm length and 1 cm internal diameter. The known amount, 0.50 g De–Oiled Soya of 0.150–0.088 mm (36 BSS Mesh) was selected. The weighed adsorbent was made into slurry with double distilled water and kept overnight and then fed slowly into the column by using glass wool support. To avoid air entrapment, adsorbent was fed slowly into the column and the water of the slurry was continuously expelled through the column outlet. The column was then loaded with dye solution 6 × 10–5 M concentration, which was eluted through the column at a constant flow rate of 0.5 mL min–1 and expelled dye solutions, each of 10 mL volume, were collected in test tubes to monitor the dye concentration by UV/Visible spectrophotometer.
Then concept of regeneration of adsorbent is important in industrial applications for the removal of dye from wastewater. Once the collected effluent concentrated solution matched the original concentration of loaded dye, column operation was shut down. In order to recover to adsorbed Rose Bengal dye from the fixed bed exhausted column, eluting dilute NaOH were passed through the column with constant flow rate 0.5 mL min–1 finally drain out adsorbed material from the column were then dried. After complete recovery of dye the column was washed with hot water and made ready for the next cycle of operation.
Result and Discussion
Characterization of adsorbent
The characterization of the activated De–Oiled Soya was carried out by conventional chemical methods. The maximum profat contains was found about 48.0% in dry sample matters in adsorbent, while proteins, moisture, fiber, SiO2, P and Ca were present 29.0,11.0, 6.0, 2.0, 0.7 and 0.2%, respectively. Other physicochemical properties like, surface area, porosity, density and loss of ignition of dry activated De–Oiled Soya were obtained as 728.6 cm2.g–1, 67%, 0.5614 g.mL–1 and 4.27%, respectively.
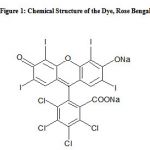
The surface structure of activated De–Oiled Soya was observed by Scanning Electron Microscope (SEM) studies. SEM photographs ascertained that the particulates of activated De–Oiled Soya are porous and almost spherical (Fig. 2). The Differential Thermogravimetry Analysis (DTA) curves of the activated De–Oiled Soya exhibit its good thermal stability and negligible weight loss was accounted. The Infra-Red spectrum of the activated De–Oiled Soya gave sharp absorption bonds at 479, 779, 1113, and 3459 cm–1, confirming thereby the presence of gorthite [4(FeO.OH)], corundum [2(α-Al2O3)], coesite [SiO2], and laumonite [4(CaAl2Si4O12.4H2O)], respectively (Fig. 3).
 |
Figure 2: SEM Photographs of the Activated De-Oiled Soya Particles |
 |
Column Studies
Fixed–bed column studies were applied in order to observe the adsorptive tendency of the adsorbent materials as introduced by Michaels10 and Fornwalt and Hutchins11. The adsorption process in fixed-bed columns appears to have a distinct advantage over batch-type operations due to the fact that in batch-type operations the effectiveness of the adsorbent material for removing solute from solution decreases as the adsorption proceeds, whereas in column operations the adsorbent is continuously in contact with solution of fixed concentration. As a result the exhaustion capacity of the column is relatively greater than that of batch capacity12.
The breakthrough curve obtained for the Rose Bengal-De–Oiled Soya system were used to calculate the values of (δ) length of the primary adsorption zone, (tx)is the total time involved for the establishment of primary adsorption zone, (tδ) time for the primary adsorption zone to move down its length, (tf) time for initial formation of primary adsorption zone, (Fm) mass rate of flow of the adsorbent, (f) fraction capacity of column and percent saturation of column at break point were calculated by using following equations.
Where, Ms is the amount of adsorbed in the adsorption zone from break point to exhaustion, Co is initial concentration of adsorbate and D is the length of column.
Column adsorption and Regeneration
To evaluate the breakthrough curve of Rose Bengal, concentration 6 × 10–5 M dye solution was passes 260 mL of dye through the column at a flow rate 0.5 mL/min. The desired amount of De–Oiled Soya was fixed into the column by the help of glass wool support, whereas the De–Oiled Soya was passes through the column 260 mL of dye (Fig.4). Hence 15.86 mg of solute was present into the solution during collecting the solution 9.42 mg dye find out in the adsorption studies of De–Oiled Soya. The adsorbed dye was found to be 6.43 mg over the 1 g of De–Oiled Soya. Different column parameters obtained during the studies are presented in Tables 1 and 2. A percentage saturation of 98.97% reveals a good affinity of Rose Bengal towards the De–Oiled Soya
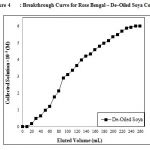 |
Figure 4: Breakthrough Curve for Rose Bengal – De–Oiled Soya Column |
Table 1: Fixed Bed Adsorber Calculations for the Adsorption of Rose Bengal in De–Oiled Soya Column
| Co
(M) |
Cx
(M) |
Cb
(M) |
Vx
(mL) |
Vb
(mL) |
(Vx – Vb)
(mL) |
Fm
(mg/cm2/min) |
D
(cm) |
| 6 × 10–5 | 4.48×10–5 | 5.88 × 10–5 | 230 | 30 | 200 | 0.0388 | 1.0 |
Table 2: Parameters for Fixed Bed Adsorber – De–Oiled Soya for Rose Bengal Adsorption
| tx
(min) |
tδ
(min) |
tf
(min) |
f | δ
(cm) |
Percentage
Saturation |
| 6691.803 | 5147.54 | 60 | 0.988 | 0.878 | 98.97 |
The desorption characteristics of Rose Bengal was best studied using a basic medium. Thus, dilute NaOH solution was allowed to percolate over the saturated adsorbents with a flow rate of 0.5 mL/min. Fig. 5 indicates that 230 mL dilute solution is needed for complete desorption of dye from De–Oiled Soya. It also reveals that about 64.50% dye was removed by eluting initial 70 mL NaOH solution on column and remaining amount of dye was recovered in next 160 mL solution. During the complete desorption process 90.93% of dye was recovered from De–Oiled Soya.
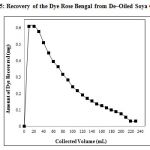 |
Figure 5: Recovery of the Dye Rose Bengal from De–Oiled Soya Column |
The adsorption efficiency of the column was calculated by reloading the column with known concentration of Rose Bengal dye. The breakthrough capacities for the first, second, third, fourth and fifth cycles were observed as 81, 73, 66, 54 and 42% for De–Oiled Soya, respectively. These results indicated that the adsorption capacity of dye over adsorbents slightly decreases with increase in adsorption – desorption cycle.
Conclusion
The experiments reveal that De–Oiled Soya proved to be efficient adsorbents for the removal of Rose Bengal dye from wastewaters. The overall research work, conclude that the adsorbent De–Oiled Soya found to have immense potential for the removal of halogen containing dyes from aqueous solutions. These adsorbent materials are cheap and easily available with high potential of adsorbing the dye from wastewaters than the commercially available material. The percentage saturation of the column was found as 98.97%, respectively. The recovery of the dye was made by eluting dilute NaOH through exhausted columns and almost 90.93% recovery of the dye was achieved at De–Oiled Soya column.
References
- “Saunders Comprehensive Veterinary Dictionary”, 3rd Edition, Elsevier, Inc., 2007.
- Sako, F., Taniguchi, N., Kobayashi, N. and Takakuwa, E., “Effects of food dyes on paramecium caudatum: toxicity and inhibitory effects on leucine aminopeptidase and acid phosphatase activity”, Toxicology and Applied Pharmacology, 39 , 1977, 111 – 117.
- Sako, F., Kobayashi, N., Watabe, H. and Taniguchi, N., “Cytotoxicity of food dyes on cultured fetal rat hepatocytes”, Toxicology and Applied Pharmacology, 54, 1980, 285 – 292.
- Tabery, H.M., “Toxic effect of rose bengal dye on the living human corneal epithelium”, Acta Ophthalmologica Scandinavica, 76, 1998, 142 – 145.
- Lee, Y.C., Park, C.K., Kim, M.S. and Kim, J.H., “In vitro study for staining and toxicity of rose bengal on cultured bovine corneal endothelial cells”, Cornea, 15, 1996, 376 – 385.
- Danylkova, N.O., Pomeranz, H.D., Alcala, S.R. and McLoon, L.K., “Histological and morphometric evaluation of transient retinal and optic nerve ischemia in rat”, Brain Research, 1096, 2006, 20 – 29.
- Kelly, K.M., Kharlamov, A., Hentosz, T.M., Kharlamova, E.A., Williamson, J.M., Bertram, E.H., Kapur, J. and Armstrong, D.M., “Photothrombotic brain infarction results in seizure activity in aging fischer 344 and sprague dawley rats”, Epilepsy Research, 47 , 2001, 189 – 203.
- Levitan, H., “Food, drug and cosmetic dyes: biological effects related to lipid solubility”, Proceedings of National Academy of Science , 74 ,1977, 2914 – 2918.
- Material Safety Data Sheet, http://www.sciencelab.com/msds.php?msdsld= 9924832.
- Michaels, A.S., “Breakthrough Curves in Ion-Exchange”, Industrial and Engineering Chemistry, 44, 1952, 1922 – 1930.
- Fornwalt, H.J. and Hutchins, R.A., “Purifying liquids with activated carbon”, Chemical Engineering Journal, 73 ,1966, 179 – 184.
- Mittal, A., Kaur, D., Malviya, A., Mittal, J. and Gupta, V.K., “Adsorption studies on the removal of colouring agent Phenol Red from wastewater using waste materials as adsorbent”, Journal of Colloid and Interface Science, 337(2) , 2009, 345 – 354.