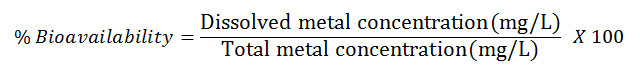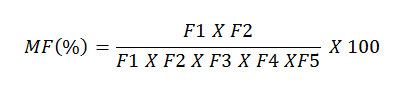Introduction
The indiscriminate dumping of degradable or non-degradable wastes into urban stream stretches and rivers has been considered one of the major causes of the unprecedented mush-rooming of heavy metals and their speciation and the results are effect on ecosystems and human health risk implications globally.
Sediments are considered to be an important carrier as well as a sink of heavy metals in the hydrological cycle (Celo et al., 1999). The majority of heavy metal emissions from anthropogenic activities accumulate in river and ocean sediments, where they are absorbed onto clays and other fine-grained materials (Ho et al., 2010).
Moreover, benthic organisms can take up metals directly from the sediments, which in turn enhance the potential of some metals entering into the food chain (Adamo et al., 2005; Chen et al., 2007).
Heavy metals can cause serious health effects with varied symptoms depending on the nature and quantity of the metal ingested (Adepoju-Bello and Alabi, 2005; Akan et al., 2010). The various ways by which heavy metals associate with various soil/sediment components determine their mobility and availability (Xian, 1987; Kabata-Pendias and Pendias, 1992; Singh, 1997; Ahumada et al., 1999).
Speciation in sediment compartment is a significant step to understand the potential environmental risk, distribution, mobility and bioavailability of pollutants. Determining the total content of heavy metals in the sediments may be useful for the characterization of pollution intensity, however, speciation of heavy metals with selective extracting agents gives further information about the fundamental reactions that govern the behaviour of metals in sediment and helps to assess the environmental impact of contaminated soil and sediment (Salomaons and Förstner, 1980; Ogunfowokan et al., 2009; Ogunfowokan et al., 2013; Leizou et al., 2015).
To assess the bioavailability of heavy metals, sequential extraction procedure consists of a series of chemical extractants, each being more drastic in action and of different nature than the previous one (Chaudhary and Banerjee, 2008; Osakwe, 2010).
Chemical speciation is a powerful and versatile technique for predicting the degree of contamination risk of a river system. This study is the first to assess the levels of heavy metals in sediments of urban stream stretches; work on speciation of heavy metals in sediments of urban stream stretches in Yenagoa City, Bayelsa State has not been reported. Therefore, due to paucity of information, it is expected that the results from this study would form a baseline data for future heavy metal pollution status in sediment of the area under study. The main objectives of this study is to investigate the speciation, elemental mobility factor and bioavailability indices of some heavy metals in sediments of urban stream stretches from Yenagoa city, Bayelsa state, Nigeria using Tessier’s five-step sequential extraction schemes.
Materials and Methods
Description of study area
Yenagoa is a non-industrialized city situated in the south-south, oil rich Niger Delta region of Nigeria. The geographical location of Yenagoa is on the north and east hemisphere. The study area lies between the coordinates of latitudes 04o15” North and latitude 05o23‟ South and longitude 05o22”West and 06o45” East. Since attaining the status of a state capital in 1996, construction and other activities have accelerated appreciably (Fig. 1).
 |
Table 1: Sites and description of activities
| Sites | Description of activities | Details |
| A | Higher institution, Residential, Dump sites , commercial, traffic, different component of vehicles and natural sources. | Agudama |
| B | Market, furniture making, Residential, commercial, traffic, different component of vehicles, and natural sources. | Tombia |
| C | Residential, dump sites , School, commercial,, traffic , different component of vehicles, natural sources | Okutukutu |
| D | Commercial, traffic, different component of vehicles, dump and natural sources. | Amarata |
Sampling and Pre-treatment of Sediment Samples
In this study, sediment samples were collected from four stations located along urban stream stretches, Yenagoa City, Nigeria. Details of these sites are given in Table 1. Sediments were sampled using a bottom grab sampler (Hydro-Bios) and then immediately transferred into plastic bags and refrigerated. In order to get a representative sample for each station, several sub-samples were collected and mixed together. The sediment samples were analyzed for total metal (UNEP, 1985) and metal speciation (Tessier et al., 1979).
Reagents Used and Their Source
All the reagents used are of analytical grade. The reagents used are HNO3 (Riedel-deHaën, Germany), 30% hydrogen peroxide, H2O2and 70% perchloric acid, HClO4 British Drug House (BDH) Chemicals Ltd, Poole, England. Solutions were prepared using double distilled water. All glass wares used (conical flask, measuring cylinder, volumetric flask, and watch glass) were washed with liquid detergent and rinsed thrice followed by oven drying.
Determination of Metal Speciation in Sediment
For the heavy metal speciation in sediment a five-step sequential extraction procedure was employed in this study. This sequential extraction was based on the principles of (Tessier et al., 1979; Abu-kukati, 2001) procedures. This extraction procedure defines the following five metal speciation regular forms: exchangeable, carbonate-bound, Fe/Mn-oxide bound, organic matter/sulfide bound and residual fraction (Table 2).
Table 2: Summary of sequential Extraction Scheme Used
| FRACTIONS | EXTRACTANT | TIME(hr) | TEMP oC | DESIGNATION |
| F1 | 1g of sediment sample +16ml of 1M MgCl2, pH=7 | 1 | RT | Soluble / Exchangeable |
| F2 | 16ml of CH3COONa/CH3COOH buffer, pH=5 | 5 | RT | Carbonate bound |
| F3 | 20ml of 0.4M NH2OH. HCl in 25% CH3COOH | 6 | 96 | Fe / Mn-oxide Bound |
| F4 | 1.3ml of 0.02M HNO3+5ml of 30% H2O2, pH=2 with HNO3 2.3ml of 30% H2O2, pH = 2 withHNO3
3. 5ml of 3.2M NH4OAc in 20% HNO3 followed by dilution to 20ml with de-ionized water |
2
3 |
85
85 |
Organic matter Bound |
| F5 | 5ml HNO3+ 10ml HF +10ml HClO4 | Residual |
*Tessier et al., (1979); **Abu-Kukati, 2001 ***RT = room temperature
Results And Discussion
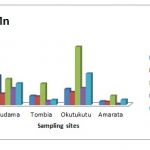
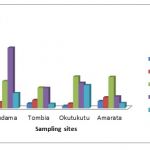
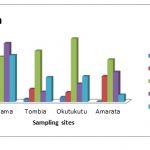
Table 3-4 Summaries the concentrations of heavy metals in (range, mean and standard deviation) expressed as milligram / Kilogram (mg/kg) of dried sediments and the operationally defined fractions are represented graphically in Figs. 2-4.
 |
 |
 |
The chemical speciation of Pb was majorly associated with the Fe/Mn-oxide fraction which ranged from 35.56 -80.76 with a mean of 52.57±19.83 mg/kg. Okutukutu stream sediment had the highest Pb concentration (80.76mg/kg followed closely by Tombia (51.06mg/kg). The next was Agudama (42.90mg/kg) and the lowest concentration of Pb was recorded at Amarata (35.56mg/kg). The concentration level in the Fe/Mn-oxide fraction of the various sites for Pb follows the pattern: Okutukutu >Tombia > Agudama > Amarata.
The Fe/Mn-Oxide fraction represents heavy metals soluble in water as well as those held by electrostatic adsorption (Osakwe, 2010); could be considered relatively stable, but could change with variations in redox conditions of the soil. The amount of metal in this phase indicated the environmental conditions of the soil. The high percentage of lead in this fraction suggests a greater contamination risk for lead (Horsfall and Spiff, 2005; Ramzan et al., 2015).
The data shows that, concentration (mg/kg) of Pb in organic/sulfide fraction ranged from 0 – 30.64 with a mean of 24.00±9.39. Agudama had the highest (30.64mg/kg), closely followed by Okutukutu (17.36mg/kg). The next was Tombia and Amarata which were not detected (ND).
Organic compounds of heavy metals can be directly or indirectly introduced into soil through the formation of complexes and can be taken up by plants. Organic content is associated with the production of gas, and in sandy soil that contains little clay, organic matter and phosphate can leach through the soil and impact ground water (Urunmatsoma and Ikhuoria, 2005; Osakwe, 2010).
Organic matter plays an important role in the distribution and dispersion of metals by mechanisms of chelating and cation exchange. This include metals associated through complexation or bioaccumulation process with various kinds of organic materials such as living organisms, detritus or coatings on mineral particles (Tokalioglu et al., 2002). Carboxyl, phenolic, hydroxyl and carbonyl functional groups are assumed to be primarily responsible for metal binding (Horsfall and Spiff, 2005; Ramzan et al., 2015).
Metals complexed with organic matter are generally described by an ion exchange, organo-metallic complexation mechanism. Metal humic complexes are reversible, and metals can be desorbed by salting out or by hydrogen ion competition (Horsfall and Spiff, 2005).
Lead is said to be non-essential major toxic metal with multiple side effects (Klassen. (1996). (Patrick et al., 1977; Klassen, 1996; McBride, 1994; EPA, 2009; Amanda, 2010) who reported that lead is an ubiquitous metal found in all environments, various phases, and all biological systems. At high pH, it is less soluble and complexes with organic matter, sorption to oxides and silicate clays, precipitate as carbonates and hydroxides. It has high affinity for Mn oxides and the least mobile metal known, however, does bio accumulate when complexed to organic matter and tends to accumulate in the roots of plants. Lead and its compounds are known to cause some medical effects such as: cardiotoxicity, neuropathy, encephalopathy, and kidney, vascular, and reproductive effects.
Sia Su, 2008 reported that the presence and potential exposures of the community to groundwater contaminants may contribute to the predilection of human health impacts, from simple poisoning to cancer, heart diseases and teratogenic abnormalities.
Next in importance for lead content are carbonate fraction, residual fraction and exchangeable fraction in sediment ranged from 0 – 22.48; 0 – 9.7 and 0 – 5.01 with mean values of 13.96±9.80, 6.45±45 and 4.66±0.45 respectively(Table 3-4). The association pattern of lead in the different geochemical fractions was in the order: Fe/Mn oxide > organic > carbonate > residual > exchangeable.
For manganese, the Fe/Mn oxide fraction was the most important fraction which ranged from 19.58 to 123.22 with an average of 60.71±44.26 mg/kg. Okutukutu had the highest (123.22mg/kg), closely followed by Agudama (54.94mg/kg), next was Tombia (45.08mg/kg) and Amarata (19.58mg/kg) respectively.
Next in importance for manganese content was the residual fraction which ranged from 10.70 to 66.58 with a mean of 33.52±27.48 mg/kg. The fraction can be taken as a guide to the degree of pollution of the soil. The smaller the percentages of the metal present in this fraction, the greater the pollution of the area (Horsfall and Spiff, 2005). The residual fraction is considered the most stable, less reactive and less bioavailable since it is occluded within the crystal lattice layer of silicates and well crystallized oxide minerals (Abeh et al., 2007; Schawarzenbach et al., 1993).
Manganese is an essential metal for plants and animals with potential for toxicity. The solubility of Mn is strongly influenced by soil potential, Eh and pH forming soluble hydroxides and carbonates above pH 7. Manganese complexes with organic matter and silicates in soils above pH 6 and usually enters the food chain through foods such as fruits and vegetables. Manganese can be adsorbed onto soil; the extent of adsorption depends on the organic content and cation exchange capacity of the soil.
Table 3: Concentrations (mg/kg) of heavy metals in various geochemical fractions
| PARAMETERS | FRACTIONS | ||||
| exchangeable | carbonate | Fe/Mn oxide | organic | residual | |
| Pb
range Min Max Mean±std |
0-5.01 0.01 5.01 4.66±0.45 |
0-22.48 0.01 22.48 13.96±9.83 |
35.56-80.76 35.56 80.76 52.57±19.83 |
0-30.64 ND 30.64 24.00±9.39 |
0.00-9.7 ND 9.70 6.45±4.45 |
| Mn
range Min Max Mean±std |
7.76-62.32 7.76 62.32 30.86±23.55 |
6.7-27.26 6.70 27.26 18.96±9.01 |
19.58-123.22 19.58 123.22 60.71±44.26 |
1.68-35.82 1.68 35.82 20.42±17.85 |
10.70-66.58 10.70 66.58 33.52±27.48 |
| Cu
range Min Max Mean±std |
0.08-0.12 0.07 0.12 0.09±0.04 |
0.08-0.18 0.08 0.18 0.10±0.05 |
0.35-0.54 0.35 0.54 0.47±0.09 |
0.20-1.06 0.20 1.06 0.51±0.38 |
0.07-0.40 0.07 0.40 0.20±0.15 |
| Zn
range Min Max Mean±std |
0.04-0.50 0.4 0.50 0.24±0.40 |
0.60-1.62 0.60 1.62 0.96±0.45 |
2.67-3.99 2.67 3.99 3.18±0.59 |
0.63-3.71 0.63 3.71 1.84±1.35 |
0.44-2.94 0.44 2.94 1.63±1.02 |
| Cr
range Min Max Mean±std |
0.00-1.84 0.00 1.84 1.20±0.91 |
ND ND ND ND |
ND ND ND ND |
0.00 0.00 25.9 20.24±8.00 |
0.00-23 0.00 23.00 22.37±0.90 |
| Cd
range Min Max Mean±std |
ND ND ND ND |
ND ND ND ND |
ND ND ND ND |
ND ND ND ND |
ND ND ND ND |
Table 4: Comparison of heavy metal concentration (mg/kg) in sediment with SQGs some river
| Pb | Mn | Cu | Zn | Cr | Cd | |
| sites | mean±std | mean±std | mean±std | mean±std | mean±std | mean±std |
| A | 16.95±18.62 | 44.46±15.30 | 0.40±0.39 | 2.16±1.42 | 16.49±12.86 | ND |
| B | 51.06±0.01 | 20.38±14.56 | 0.20±0.14 | 1.28±1.19 | 0.54±0.01 | ND |
| C | 31.19±33.94 | 57.36±39.82 | 0.30±0.23 | 1.52±1.47 | 7.89±9.40 | ND |
| D | 16.62±13.44 | 9.36±6.63 | 0.23±0.18 | 1.32±1.09 | 23.00±0.01 | ND |
| MIN | 16.62±13.44 | 9.36±6.63 | 0.20±0.14 | 1.28±1.19 | 0.54±0.01 | ND |
| MAX | 51.06±0.01 | 57.36±39.82 | 0.40±0.39 | 2.16±1.42 | 23.00±0.01 | ND |
| MEAN | 28.96±16.50 | 32.89±19.08 | 0.28±0.24 | 1.57±1.29 | 11.98±5.57 | ND |
| TEL | 35 | – | 35.7 | 123 | 37.3 | 0.596 |
| LEL | 31 | – | 16 | 120 | 26 | 0.6 |
| ERL | 35 | – | 70 | 120 | 80 | 5 |
| TEC | 35.8 | – | 31.6 | 121 | 43.4 | 0.99 |
TEL =Threshold effect level, LEL = Lowest effect level, ERL= Effect range low (Smith et al. 1996), Persaud et al. 1993, (EC and MENVIQ 1992), Long and Morgan 1991; MacDonald et al., 2000)
It can bioaccumulate in lower organisms (e.g., phytoplankton, algae, molluscs and some fish) but not in higher organisms; biomagnification in food chains is not expected to be very significant (Abbasi et al., 1998; Eaton, 2005; WHO, 2004; Akan et al., 2010). It is also being introduced into the environment as an octane booster in gasoline, replacing Pb in the 1980’s. Manganese compounds are used in products such as batteries, glass and fireworks, as an oxidant for cleaning, bleaching and disinfection purposes, fertilizers, varnish and fungicides and as livestock feeding supplements. Common effects of excessive Mn are pneumonitis, epithelial necrosis, mononuclear proliferation, central nervous system and liver disorders, and neuropsychiatric disorder (Patrick et al., 1977; Klassen, 1996; McBride, 1994; EPA, 2009).
Other important fractions controlling the mobility and bioavailability of heavy metals in sediment include: exchangeable, carbonate, organic matter and sulfides which ranged from 7.76 – 62.32; 6.70 – 27.26 and 1.68 – 35.82 with mean values of 30.86±23.55, 18.96±9.01 and 20.42±17.85 respectively (Table 3-4). The association pattern of manganese in the different geochemical fractions was in the order: Fe/Mn oxide > residual > exchangeable >organic > carbonate.
The speciation results from the present study found only small amount of copper associated with the five geochemical fractions (Table 3-4). The concentration levels in the fractions followed the order: Organic (0.20-1.06, 0.51±0.38 mg/kg) > Fe/Mn-oxide (0.35-0.54, 0.47±0.09 mg/kg)> residual (0.07-0.40, 0.20±0.15mg/kg) > carbonate (0.08-0.18, 0.13±0.05mg/kg) > exchangeable (0.08-0.12, 0.09±0.04mg/kg). The strong association between soil Cu and the organic matter is also in agreement with the general findings that Cu forms stable complexes with soil humus (Stumm and Morgan, 1981; Chengo et al., 2013).
The residual fraction is a major carrier of metals in most environmental systems and can be taken as a guide to the degree of non-availability of metals (Horsfall and Spiff, 2005). As a result of weathering, a fraction of the trace constituent content is gradually transferred to forms accessible to plants (Hlavay et al., 2004). In all the samples analyzed, Cu was found to be mostly associated with the residual fraction and the results are concomitant with those reported by (Yobouet et al. 2010; Ramzan et al., 2015). In comparison with carbonate minerals, Fe-Mn oxide minerals have relatively large area and surface site density. The Fe-Mn oxide, the reducible phase of the soil under oxidizing conditions is a significant sink for the heavy metals (Forstner and Wittmann, 1981).
The chemical speciation results from the present study, also found only small amount of zinc associated with the five geochemical fractions and was highest in the Fe/Mn-oxide fraction which ranged from 2.67-3.99 with a mean value of 3.18±0.59mg/kg, while the exchangeable fraction had the lowest which ranged from 0.04-0.50 with a mean value of 0.24±0.35mg/kg (Table 3-4).
The sequence of concentration levels of zinc in the different geochemical fractions is as follows; Fe/Mn oxide (2.67-3.99, 3.18±0.59mg/kg)> organic (0.63-3.71, 1.84±1.35mg/kg) >residual (0.44-2.94, 1.63±1.02ng/kg) > carbonate (0.60-1.62,0.96±0.45mg/kg) > exchangeable (0.04-0.50,0.24±0.35mg/kg).
In comparison with carbonate minerals, Fe-Mn oxide minerals have relatively large area and surface site density (Forstner and Wittmann, 1981). The Fe-Mn oxide, the reducible phase of the soil under oxidizing conditions is a significant sink for the heavy metals. Organic matter plays an important role in the distribution and dispersion of metals by mechanisms of chelating and cation exchange. This includes metals associated through complexation or bioaccumulation process with various kinds of organic materials such as living organisms, detritus or coatings on mineral particles (Tokalioglu et al., 2002; Ramzan et al., 2015). Carboxyl, phenolic, hydroxyl and carbonyl functional groups are assumed to be primarily responsible for metal binding.
Zinc is said to be in the category of essential metals with potential for toxicity. Zinc is used in a number of alloys including brass and bronze, batteries, galvanizing steel and iron products, pesticides, fungicides and pigments.
Excessive intake of Zn may lead to vomiting, dehydration, abdominal pain, nausea, lethargy and
Dizziness (ATSDR, 1994). Zinc is an essential element for plants and animals growth but at elevated levels it is toxic to some species of aquatic life (WHO, 2004). Furthermore, it is involved in a variety of enzyme systems which contribute to energy metabolism, transcription and translation. Excessive intake of Zn may lead to vomiting, dehydration, abdominal pain, nausea, lethargy and dizziness (ATSDR, 1994). Zinc is also potentially hazardous and excessive concentrations in soil lead to phytotoxicity as it is a weed killer (Anglin-Brown et al., 1995; Abbasi et al., 1998; WHO, 2004).
Contaminants are bound to various forms of organic matter and these bonds are strong. These contaminants are released when the oxidizing environment changes, degrading the organic matter. Contamination in the Organic fraction is the least biologically available (Tessier et al., 1979).
The chemical speciation of Cr was majorly associated with the organic fraction which ranged from 0 -25.90 with a mean of 20.24±8.00 mg/kg. The sequence of concentration levels of Cr in the different geochemical fractions is as follows; organic (0-25.9, 20.24±8.00mg/kg)> residual (0-23, 22.37±0.90ng/kg) > exchangeable (0-1.84, 1.2±0.91mg/kg) > carbonate (ND) and Fe/Mn-oxide (ND).
Chromium is a major toxic metal with oxidation states ranging from Cr (II) to Cr (VI), however, only the Cr (III), and Cr (VI) states are environmentally available, primarily from the discharge of industrial wastes. The Cr (III) is considered a minor problem, while Cr6+ is very toxic and carcinogenic (ATSDR, 2000; Akan et al., 2010; Amanda, 2010). Chromium (III) had been described as an essential nutrient that helps the body use sugar, protein, and fat (Hati et al., 2005). Some of the medical effects chromium includes eczematous dermatitis, cause cancer of the lungs, nasal cavity and paranasal sinus, cancer of the stomach and larynx (ATSDR, 2000).
The speciation results from the present study found no amount of Cd associated with any of the five geochemical fractions except fraction four (organic) of Agudama (Table 3-4).This could be as result of the non-industrialization of the city. Cadmium is also a major toxic metal with multiple side effects. It’s 2+ state sorbs weakly to organic matter, clays, and oxides at pH below 6 and may be released into the environment with a change in ionic composition of the pore waters. It is commonly used in batteries, paints and plastics, and most biological exposure comes from food.
Bioavailability
The bioavailability of metals expressed in percentage ( was calculated as dissolved metal concentration via analysis of filtered water samples divided by the total metal concentration of unfiltered water samples. The term “bioavailability” is meant to denote heavy metals in a water-soluble form that plant and animal communities can readily uptake and assimilate (Kaviraj and Das, 1999; Desta et al., 2012). The bioavailability is mathematically expressed as follows:
The percentage bioavailability (Table 5) of the metals revealed a maximum and minimum value for Cr (87.87%) and Cu (37.91%) respectively. Mido and Satake, 2003 reported that the bioavailability of the heavy metals depend on the concentration of anions, chelating ligands, pH and Redox status and the presence of absorptive sediments. These findings are in agreement with those reported by Osakwe, 2010; Desta et al., 2012.
Table 5: % Bioavailability for different heavy metals in water
| sample | parameter | Heavy metals | |||||
| Pb | Mn | Cr | Cd | Cu | Zn | ||
| Filtered | mean | 0.187 | 0.432 | 0.210 | 0.087 | 0.055 | 0.342 |
| Unfiltered | mean | 0.219 | 0.494 | 0.239 | 0.141 | 0.173 | 0.390 |
| Bioavailability | 85.39 | 87.45 | 87.87 | 61.70 | 37.91 | 87.69 | |
Mobility Factor (MF)
In a five-step sequential extraction scheme, the mobility factor was calculated as the concentrations of metal in easily remobilizable fractions (F1 + F2) divided by the combine concentrations in all the five operationally defined geochemical fractions(F1 +F2 + F3 + F4 +F5) (Salbu et al., 1998; Narwal et al, 1999; Kabala and Singh, 2001). The Mobility Factor (MF)
is mathematically expressed as follows:
Where, F1 = adsorptive and exchangeable fraction; F2 =carbonate fraction; F3 = Fe-Mn oxide fraction; F4 = organic fraction and F5 = residual fraction. The mobility factors (MF) of the metals for all the sites are presented on Table 6.
Table 6: Mobility factors ( )of the metals in the samples
|
Locations |
Heavy metals | |||||
| Pb | Mn | Cu | Zn | Cr | Cd | |
| A | 9.46 | 38.74 | 11.33 | 12.08 | 3.72 | 0.00 |
| B | 0.00 | 36.79 | 22.08 | 15.47 | 1.00 | 0.00 |
| C | 21.35 | 21.34 | 7.72 | 11.63 | 0.00 | 0.00 |
| D | 31.92 | 30.90 | 26.95 | 24.53 | 0.00 | 0.00 |
The mobility factor (MF) values of the metals in all the sites and profiles follow the order Mn>Cu>Zn>Pb>Cr>Cd. The high present of manganese in the sediment could be attributed to dumping wastes in the streams.
Karczewska 1996; Ma and Rao 1997; Ahumuda et al. 1999; Narwal et al. 1999; Kabala and Singh 2001 who reported a high MF value for heavy metals in soil has been interpreted as evidence of relatively high lability and biological availability.
According to the reports of previous workers Narwal et al., 1999; Kabala and Singh, 2001; Osakwe, 2010; Osakwe et al., 2014 a high mobility factor (MF) value for metal in sediment is an indication of relatively high mobility and biological availability.
Conclusion
A five-step sequential extraction method was employed to investigate selected heavy metal in sediments from urban stream stretches of the Yenagoa City, Bayelsa State, Nigeria. The concentrations of the heavy metals in sediment from urban stream stretches were lower than the limits set by SQGs consensus-based TEC (Threshold Effect Concentrations) values. This is of important considering that heavy metals are toxic, persistent and their bioaccumulation leads to serious effect on the ecosystem and human health risk. Pb and Mn in sediment prevail mostly in exchangeable, carbonate and Fe/Mn-oxide fractions. Cu, Zn and Cr were more prevalent in organic and residual fractions, while Cd was below detection limit except for the organic fraction at agudama. Assessment of the sediments using the mobility factor and percentage bioavailability reveals the following sequence: Mn >Cu>Zn >Pb >Cr>Cd and Cr>Zn>Mn>Pb>Cd>Cu respectively. The ecotoxicological sense of heavy metal contamination in sediments was determined using sediment quality guidelines developed for metals in freshwater ecosystems reveals that the studied heavy metals in sediments of the Yenagoa City, urban stream stretches do not exceed consensus-based TEC values which can lead to adverse impact on the ecosystem and health issues. The results further reveal that the heavy metals in sediments of the urban stream stretches are accumulating appreciably. Consequently, therefore, it is expected that regulatory authorities should be encouraged to institute environmentally friendly Frame-work or interventions to maintain the immerse biodiversity, aesthetic value and reduce anthropogenic discharges and indiscriminate dumping of wastes into the urban streams.
References
- Abbasi, S. A., Abbasi, N, and Soni, R., (1998). Heavy Metals in the Environment, 1st Edn. Mittal Publications, ISBN: 81-7099-657-0, p.314.
- Abeh, T., Gungshik, J., and Adamu, M. , (2007). Speciation Studies of trace elements levels in sediments from Zaramagada stream in Jos, PlateauState, Nigeria. J. Chem. Soc. Nig. 32(2) 218-225.
- Abu-kukati, Y., (2001). Heavy metal distribution and speciation in sediments from ziglab dam-Jordan. Geol. Engineering. 25:33-40.
- Adamo P., Arienzo, M., Imperato, M., Naimo, D., Nardi, G., and Stanzione, D., (2005). Distribution and partition of heavy metals in surface and sub-surface sediments of Naples city port. Chemosphere, 61: 800–80.
- Adepoju-Bello, A. A., and Alabi, O. M., (2005). Heavy metals: A review. The Nig. J. Pharm., 37: 41-45.
- Ahumada, I., Mendoza, J., and Ascar, L., (1999): Sequential extraction of heavy metals in soils irrigated with wastewater. Commun. Soil Sci. Plant Anal. 30: 1507 – 1519.
- ATSDR, Agency for Toxic Substances and Disease Registry, (1994). Toxicological profile for zinc. US Department of Health and Human Service, Public Health Service, (205):-88-0608.
- ATSDR, Agency for Toxic Substances and Disease Registry, (2000). Toxicological Profile for Chromium. Atlanta, GA: U.S. Department of Health and Human Service, Public Health Service. 1600 Clifton Road N.E, E-29 Atlanta, Georgia 30333 (6-9): 95-134.
- Akan, J. C., Abdulrahman, F. I., Sodipo, O. A., Ochanya, A. E., and Askira, Y. K., (2010). Heavy metals in sediments from River Ngada, Maiduguri Metropolis, Borno State, Nigeria. J. Environ. Chem. Ecotoxicol. 2(9), 131-140.
- Amanda, J. Z., (2010). Speciation of heavy metals in disturbed and undisturbed sediments from Atchafalaya Bay, Houma Navigation Canal, and Southwest Pass; Louisiana , M. Sc. Thesis, Department of Geology and Geophysics, B.S. University of Hawai‟i Hilo. 2-8
- Anglin-Brown, B., Armour-Brown, A., Lalor, G. C., (1995). Heavy metal pollution in Jamaica 1: Survey of cadmium, lead and zinc concentrations in the Kintyre and hope flat districts. DOI: 10.1007/BF00146708. Environ. Geochem. Health, 17: 51-56.
- Celo, V., Babi, D., Baraj B., and Cullaj, A., (1999). An assessment of heavy metal pollution in the sediments along the Albanian coast. Water, Air, and Soil Pollution, 111: 235–250. Kluwer Academic Publishers.
- Chaudhary, S., and Benerjee, D. K., (2008). Speciation of some heavy metals in coalfly ash. Chem. Spec..Bio 19(3): 95-102.
- Chen, C. W., Kao, C. M., Chen, C. F., and Dong, C. D., (2007). Distribution and accumulation of heavy metals in the sediments of Kaohsiung Harbor, Taiwan. Chemosphere, 66: 1431–1440.
- Chengo, K., Murungi, J., and Mbuvi, H., (2013) . Speciation of Zinc and Copper in Open-Air Automobile Mechanic Workshop Soils in Ngara Area-Nairobi Kenya. Resources and Environment 3(5): 145-154 DOI: 10.5923/j.re.20130305.06.
- Desta, M. B., Asgedom, A. G., and Gebremdhin, W., (2012). Health Risk Assessment Of Heavy Metals Bioaccumulation In Water, Sediment And Three Fish Species (Labeobarbus Spp, Clarias Gariepinus And Oreochromis Niloticus) Of Tekeze River Dam, Tigray, Northern Ethiopia, Journal of Atmospheric and Earth Environment, 1(1) pp.19-29.
- Eaton, A.D., (2005). Standard Methods for the Examination of Water and Waste Water. 21st Edn. American Public Health Association, Washington, ISBN. 0875530478. pp. 343-453.
- Environmental Protection Agency, (2009). National Primary drinking Water Regulations. EPA 816-F-09-0004.
- Forstner, U., and Wittmann, G. T., W. (1981). Metal Pollution in the Aquatic Environment. New York, Springer-Verlag
- Hati, S. S., Joseph, C. A., and Ogugbuaja, V. O., (2005). Comparative assessment of chromium discharge in Tannery wastewater in Kano State, Northern Nigeria. Proceed. Of the 28th Ann. Int. Confer. CSN 2 (1): 137-139.
- Ho, H. H., Swennen, R., and Damme, A.V., (2010). Distribution and contamination status of heavy metals in estuarine sediments near Cua Ong Harbor, Ha Long Bay, Vietnam. Geologia Belgica. 13/1-2: 37-47.
- Hlavay, J., Prohaska, T., Weisz, M., Wenzel, W. W., and Stingeder, G. J., (2004). Determination of trace elements bound tosoils and fractions.Environmentalist 26,123-128.
- Horsfall, M Jnr., and Spiff, A. I., (2005). Speciation and bioavailability of heavy metals in sediment of Diobu River, Port Harcourt, Nigeria. Europ. J. Sci. Res. 6(3): 20-36.
- Kabala, C., and Singh, B. R., (2001). Fractionation and Mobility of Copper, lead, and zinc in Soil Profile in the vicinity of a Copper Smelter, J. Environ. Qual. 30:485-495.
- Kabata-Pendias, A., and Pendias, H., (1992). Trace element in sols and plant, CRC Press, Boca Raton, FL. Am. J. 34: 231 – 237.
- Karczewska, A., (1996). Metal species distribution on top and subsoil on an area affected by smelter emission. Appl. Geochem. 11: 35-42.
- Kaviraj, A., and Das, S., (1999). Effects of fertilization on the deposition, partitioning and bioavailability of copper, zinc and cadmium in four perennial ponds of an industrial town, Indian Journal of Environmental Health 41, 6–15.
- Klassen, C. D., (1996). Casarett and Doull‟s toxicology: The basic science of poisons 5th edition. Mc Graw Hill pp. 1111.
- Leizou, K. E., Horsfall, M. J., and Spiff, A. I., (2015). Speciation of some heavy metals in sediments of the Pennington River, Bayelsa State, Nigeria. American Chem. Sci. Journ. ACSj, 5(3): 238-246.
- Long, E. R., and Morgan L.G., (1991) The potential for biological effects of sediment-sorbed contaminants tested in the National Status and Trends Program. NOAA Technical Memorandum NOS OMA 52, National Oceanic and Atmospheric Administration, Seattle, WA, 175 pp 1 appendices
- Ma, L. , and Rao, N., (1997). Chemical fractionation of cadmium, copper, nickel and zinc in contaminated soils. J. Environ. Qual. 26: 259-264.
- McBride, M. B., (1994). Environmental chemistry of soils. Oxford University Press. pp 406.
- MacDonald, D. D., Ingersoll, C. G., and Berger, T. A., (2000). Development and Evaluation of Consensus-Based Sediment Quality Guidelines for Freshwater Ecosystems. Environ. Contam. Toxicol. 39, 20–31. DOI: 10.1007/s002440010075.
- Mido and Satake. (2003). Chemicals in the environment. In Toxic metals, eds. S. Sethi and S.A. Iqbal, 45–68. New Delhi: Discovery Publishing House.
- Narwal, R. , Singh, B. R., and Selbu, B., (1999). Association of Cadmium, Zinc, Copper. And Nickel with Components in Naturally Heavy Metal Rich Soils Studied by Parallel and Sequential Extraction. Commun. Soil Sci. Plant Anal., 30, 1209 – 1230.
- Ogunfowokan, A. O., Oyekunle, J.A.O., Durosinmi, L.M., Akinjokun, A.I., and 0Gabriel, O.D. (2009). Speciation study of lead and manganese in roadside dusts from major roads in Ile-Ife, South Western Nigeria. Chemistry and Ecology, 25(6): 405 – 415.
- Ogunfowokan, A.O., Oyekunle, J.A.O., Olutona, G.O., Atoyebi, A.O., and Lawal A., (2013). Speciation Study of Heavy Metals in Water and Sediments from Asunle River of the Obafemi Awolowo University, Ile-Ife, Nigeria. Intern. J. Environ.Protect., 3(3), pp. 6-16.
- Osakwe, S. A., (2010). Chemical Speciation and Mobility of Some Heavy Metals in Soils around Automobile Waste Dumpsites in Northern Part of Niger Delta, South Central Nigeria. Appl. Sci. Environ. Manage. 14 (4) 123 – 130.
- Osakwe, J. O., Adowei, P. and Horsfall Jnr. M., (2014). Evaluation of heavy metal species in bottom sediments from Imo River system, South-Eastern Nigeria. Research Journal of Chemical Sciences. 4(6), 23-30.
- Patrick, W.H., Gambrell, R.P., and Khalid, R.A., (1977). Physiochemical factors regulating solubility and bioavailability of toxic heavy metals in contaminates dredge sediment. J. Environ. Sci. Health. A12: pp.475 – 492.
- Persaud, D., Jaagumagi, R., and Hayton, A., (1993). Guidelines for the protection and management of aquatic sediment quality in Ontario. Water Resources Branch, Ontario Ministry of the Environment, Toronto, 27 pp.
- Ramzan, S., Bhat, M. A., Wani, M. A., Raza,M., and Akhtar, S. (2015). Speciation of Nickel, Lead and Cadmium under Different Soil Land Uses of Lesser Himalayas. IJREAS.5(7),72-98.
- Salbu, B., Kreling, I., and Oughlon, D.H., (1998). Characterization Radioactive particles in the environment, Analyst, 123, 843-849.
- Salomaons, W., and Förstner, U., (1980). Trace metal analysis of polluted sediments. Part 2. Evaluation of environmental impact. Environ. Technol. Lett. 1: 506 – 517.
- Schwarzenbach R. P., Gschwend P.M., and Imboden D. M., (1993). Environ. Org. Chem. John Wiley and Sons Inc., New York. 259-261.
- Singh, B. R., (1997). Soil Pollution and contamination in R. Lal (ed.), Methods of Assessment of Degradation, CRC Press, Boca Raton, FL. 279 – 299.
- Smith, S. L., MacDonald D.D., Keenleyside K. A., Ingersoll C. G., and Field J., (1996). A preliminary evaluation of sediment quality assessment values for freshwater ecosystems. J Great Lakes Res 22:624–638.
- Stumm, W., and Morgan, J. J., (1981). Aquatic chemistry: An introduction emphasizing chemical equilibria in natural waters. 2nd ed. John Wiley & Sons, New York.
- Su, G.L.S., (2008). Assessing the Effect of a Dumpsite to Groundwater Quality in Payatas, Philippines. American J. Environ. Sci. 4, pp. 276-280.
- Tessier, A., Campbell, P.G.C., and Bisson, M., (1979). Sequential extraction procedure for the speciation of particulate trace metals Anal. Chem., 51, 844– 851.
- Tokalioglu, S., Kartal, S., and Elc, L., (2002). The use of extractants in studies on trace metals in soils. Analytical Chimica Acta 33, 410-413.
- UNEP, (1985). Reference methods for marine pollution studies, United Nations Environment Program. Regional seas. 31-39.
- Urunmatsoma, S. O., Ikhuoria, E. U., (2005). Effect of leachates (heavy metal content) from solid waste at “Effurun roundabout dumpsite” Warri, Nigeria. Chem.. Tech. J. 1: 195-202.
- WHO, (2004). Guidelines for Drinking Water Quality. 3rd Edn. World Health Organization, ISBN: 92- 4-154638-7. p. 516.
- Xian, X., (1987). Chemical partitioning of cadmium, zinc, lead and copper in soils near smelters. J. Environ. Sci. health, A6: 527 – 541.
- Yobouet, Y. A., Adouby, K., Trokourey, A., and Yao, B., (2010). Cadmium, copper, lead and zinc speciation in contaminated soils.Int. J. Eng. Science and Tech.2, 802-812.