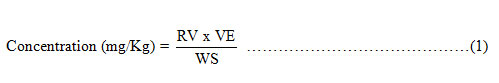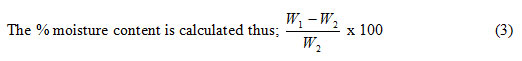Introduction
Jute Mallow (Corchorus olitorius) is a popular leafy vegetable that is cultivated in the dry, semi-arid and humid regions of Africa primarily for its nutritional value1. It is a rich source of iron [and therefore recommended for pregnant women and nursing mother2, protein, calcium, thiamin, riboflavin, niacin, folate, and dietary fibre3. The vegetable is called several names in different countries, including Jews mallow or Jute mallow in English, Egyptian spinach, and bush okra in South Africa3. It is also known in Nigeria as “ayoyo” in Hausa, “ewedu” in Yoruba and “ahuara” in Igbo4,5.
It has been reported that jute mallow is demulcent, emollient, diuretic, cleansing, and tonic in nature (Fasinmirin, 2009). The plant also has numerous other uses such as: in Kenya to treat toothache; in Congo against heart troubles; in Tanzania an infusion from the leaves is taken to treat constipation, and the seeds are taken as a purgative in Nigerian1.
However, as important and useful as this vegetable is, especially for its nutritional and medicinal applications, the presence of any toxic metals in it poses a serious health risk both to the plant itself and the consuming animals including man. Although some metals are regarded essential nutrients to plants, their presence in excess concentrations can lead to various toxic effects such as oxidative stress and inhibition of enzyme activities7.
These toxic metals constitute a serious threat to the well-being of man, wildlife and even plants if present above certain threshold levels8. The World Health Organization (WHO) has classified cadmium, copper, zinc, iron, mercury and lead as metals of serious concern9. Though, zinc is an essential element which is important in the physiological functions of living tissues and regulates many biochemical processes when present in the trace concentrations10. Concentrations of Zinc above the threshold level can cause several toxic effects such as reduction in chlorophyll content and the rate of photosynthesis in plants11, 12. Kidney dysfunction, hepatic damage and hypertension are some of the adverse effects of cadmium on health cadmium. It is also implicated in cancer and genetic changes in humans13.
The high level of awareness about the harmful effect of the metals has generated serious concern among the populace and governments at various levels14. It is therefore very important to monitor the levels of heavy metals in the environment, because of the great concern associated with contamination of soil – water – plant ecosystem with these toxic metals and their possible influence on the food chain which eventually has adverse effects on human health. This has led to several studies on the levels of toxic and potentially harmful metals in numerous environmental samples including food items. This study investigates the concentration, possible accumulation and distribution of cadmium and zinc in different parts of jute mallow irrigated with aqueous solutions of the metals. A major source of heavy metals in the environment is the discharge of untreated industrial effluents into rivers and lakes which are the major sources of water for irrigation.
Material And Methodology
Analytical reagent (AR) grade chemicals and distilled water were used throughout the study.
Preparation of Heavy metals concentration
A stock solution was prepared for both metals; 100 ppm stock was prepared for cadmium while 1000 ppm stock was prepared for the zinc. After which solutions of varying concentrations were prepared for both metals by diluting the stocks.
Preparation of soil for planting
Sandy-loam was used as the soil for planting the vegetable which was collected on the same location and at the same time. It was then thoroughly mixed (to ensure an even distribution of the soil nutrient) and sieved to remove large particles for proper aeration and water percolation.
Planting Processes
Seeds of jute plant (Corchorus olitorius), (NHCO-2 cultivar collected from the National Horticultural Research Institute, NIHORT, Ibadan), were tied in a clean cloth, steeped into boiling water for about 5 – 10 seconds (to break dormancy) and were later sown in a ready prepared nursery bed to germinate. Two weeks after sowing, at three to four leaf emergence stages, the seedlings were transplanted into the pots at the rate of three seedlings/pot. At three weeks after transplanting, heavy metals were applied according to the concentrations of each metal prepared. Wetting with ordinary water was done consequently until it begins to germinate.
The plant pots were arrayed into three groups based on the metal (Cd and Zn) concentrations to be applied. The first group was cadmium application only – at the concentrations of 0, 10, 20 and 40 ppb. The second group was zinc application only – at the concentrations of 0, 20, 40 and 80 ppm. And the third group was a binary application of the metals – at concentrations of 0, Zn/Cd-40ppm/10ppb, Zn/Cd-40ppm/20ppb, Zn/Cd-40ppm/40ppb. The concentration of the zinc was kept constant at 40 ppm while cadmium varied at 10, 20 and 40 ppb. All the pots were labelled according to the concentration of the metals applied. The application of these concentrations was done weekly for four consecutive times.
Measurement of Parameters
Three parameters were determined for this work; the growth, percentage moisture content and metal concentration using Atomic Absorption Spectrophotometer (AAS).
In determining the growth parameter, the shoot length was measured weekly with the aid of a measuring tape and the stem width was measured using a Venier Caliper.
Percentage moisture content was determined on the whole vegetable system. After it was carefully uprooted, it was rinsed with distilled water, air dried, then weighed and recorded. This stands for the fresh weight. It was then oven dried at 80 0C to a constant weight and recorded. The last parameter, i.e. heavy metal concentration was determined using AAS by firstly digesting the dried samples with HNO3 and HClO4 mixture.
Digestion Procedure
A 0.5 g ground dried sample from each plant part of the vegetable were weighed and transferred to 150 ml conical flask followed by adding 10 ml di-acid mixture of 1:4 (Perchloric acid and Nitric acid) and thereafter kept overnight for partial digestion. The partially digested samples in conical flasks were kept on hot plate for complete digestion. After digestion, the samples were diluted with distilled water, filtered (Whatman No. 42) and made up to 50 ml in volumetric flasks. The samples were analyzed for the zinc and cadmium metals concentration using Atomic Absorption Spectrophotometer (Thermo-S4 Spectrometer) and kept in plastic bottles as per the procedure described by15.
Evaluation of Heavy metal concentration in the samples after A.A.S. analysis
The readings were taken from the equipment at wavelength 213.90 nm (Zinc) and 228.80 nm (Cadmium), then the results were converted to actual concentration of metals in samples using the equation;
Where RV = Read Value is the value of the reading obtained from the A.A.S equipment (mg/L); VE = Volume of extract is the final volume of the digest used for spectrophotometric analysis (mL); WS = Weight of sample is the weight of the sample digested (g).
Data analysis
The daily intake of metals (DIM) can be calculated by the following equation:
Where [M], K, I and W represent the heavy metal concentrations in plants (mg/kg), conversion factor, daily intake of vegetables and average body weight, respectively. The conversion factor used to convert fresh green vegetable weight to dry weight was 0.085, as described by16.
The average adult and child body weights were considered to be 55.9 and 32.7 kg, respectively, while average daily vegetable intakes for adults and children were considered to be 0.345 and 0.232 kg/person/day, respectively, as reported in the literature17.
% MC is the moisture content of the samples before analysis;
Where W1 = fresh weight of sample (g), W2 = dry weight of the sample (g)
Results and Discussion
Table 1: Mean values of some physical parameters to show the effect of Zn and Cd on the growth of jute mallow (Corchorus olitorius)
| METAL/CONC | GROWTH
|
% MC | FRESH WT (gplant-1) | ||
| Shoot length (cmplant-1) | Stem width (cmplant-1) | Root length (cmplant-1) | |||
| CONTROL | 20.31±2.31 | 0.32±0.03 | 15.08±4.26 | 76.26 | 28.73±1.65 |
| Cd – 10 ppb | 23.13±0.40 | 0.34±0.01 | 16.90±0.75 | 76.28 | 34.65±1.76 |
| Cd – 20 ppb | 22.50±0.72 | 0.34±0.02 | 17.10±1.93 | 72.36 | 32.35±1.62 |
| Cd – 40 ppb | 20.36±0.89 | 0.35±0.00 | 14.80±1.94 | 72.15 | 32.25±1.30 |
| Zn – 20 ppm | 24.85±2.00 | 0.37±0.04 | 17.82±0.54 | 73.64 | 42.85±2.87 |
| Zn – 40 ppm | 22.33±0.57 | 0.35±0.00 | 19.88±2.47 | 72.36 | 41.40±1.26 |
| Zn – 80 ppm | 19.61±4.11 | 0.35±0.04 | 16.09±0.88 | 69.97 | 32.70±0.92s |
| Zn+Cd 10 ppb | 24.85±2.00 | 0.35±0.04 | 14.34±4.72 | 70.38 | 30.45±0.98 |
| Zn+Cd 20 ppb | 22.34±0.57 | 0.36±0.00 | 17.72±1.67 | 71.29 | 37.85±1.60 |
| Zn+Cd 40 ppb | 19.61±3.23 | 0.38±0.04 | 17.90±0.18 | 74.41 | 38.25±1.70 |
The values for cadmium and zinc show zinc being kept at constant concentration of 40 ppm.
Table 1 shows the growth of jute mallow before contamination and after contamination. From the results, when contaminated with 10 and 20 ppb of Cd, the root and the shoot length increased while there was no significant difference in the stem width with that of control but at 40 ppb level of contamination with Cd, there was no significant difference in the length of the three parts to that of control. This may be because as the concentration increases the Cd moves to more edible part of the plant especially the leaves which can be evident from the fresh weight measurement presented in the table which is more than that of the control..
The results for zinc is different because at 20 and 40 ppm, all the three parts are much higher than the control which may be due to bioaccumulation but at 80 ppm, the stem width and the root length are slightly higher than that of the control while there was no significant difference between the shoot length with that of the control. The results for the metal mixture are the same as that of zinc which may imply that the Zn portion was not affected by the introduction of Cd. This is contrary to what was observed by13 and18 where introducing contaminated water led to decrease in the root and shoot length of vegetables.
Table 2: Concentrations of cadmium metal (mg Kg-1) found in the jute mallow parts
(Mean value ± SD)
| Control | Cd-10 ppb | Cd-20 ppb | Cd-40 ppb | |
| LEAF | n. d. | 0.475±0.04 | 0.315±0.03 | 2.650±0.47 |
| STEM | 13.860±0.57 | n. d. | 29.320±1.52 | 0.685±0.10 |
| ROOT | n. d. | 0.430±0.05 | 0.210±0.07 | n. d. |
The concentration of Cd in three major parts; leaf, stem and root both in the control and simulated irrigation wastewaters are presented in Table 2. The results showed that the most edible parts which is the leaf has the potential to accumulate the Cd when introduced by any source. This is evident from there markedly increase in the concentration of Cd as the wastewater being introduced also increased but with the stem there was an increase from after the introduction of 20 ppb Cd and a sharp decrease at 40 ppb Cd.
The root seems not to retain the Cd because the concentration was reducing even at higher concentrations of wastewater. This is similar to what19 observed where the leafy vegetables accumulated cadmium more than the root vegetables
Table 3: Concentrations of zinc metal (mg Kg-1) found in the mallow parts (Mean value±SD)
| Control | Zn-20 ppm | Zn-40 ppm | Zn-80 ppm | |
| LEAF | 8.120±1.05 | 38.515±1.70 | 22.265±0.18 | 46.590±1.64 |
| STEM | 19.540±3.33 | 19.995±0.34 | 33.145±0.54 | 43.415±0.21 |
| ROOT | 5.580±0.60 | 20.215±1.50 | 30.870±1.34 | 56.035±4.05 |
The results presented on Table 3 were the concentrations of zinc metal in leaf, stem and root of jute mallow planted under normal conditions and the ones irrigated with simulated wastewater of different concentrations. The results showed that all the parts accumulated zinc but the roots accumulated it most evident from the results where the concentration of zinc in control rose from 5.58 to 56.035 mgKg-1. 20 also observed this whereby the stem of spinach vegetables has higher retention for zinc.
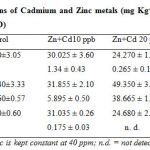 |
In Table 4, the concentrations of Cd in the control experiment and when the plants were irrigated with the aqueous mixture of the metals (Zn and Cd) are presented. From the results, the stem seems to accumulate both metals but Zn was the most accumulated as it increased from 19.54 in control to 49.35 mgKg-1 but it was a different trend both in leaf and root.
In the leaf, the concentration of Zn rose 8.120 to 30.025 mgKg-1 in the first introduction of the metal mixture solution but it was decreasing as the concentration of Cd was increasing. This may be that as the Cd concentration is increasing the translocation rate of Zn was been reduced which is evident from the results of Cd which was increasing at increased concentrations of Cd in the mixture. This trend was also evident in the root part where Zn concentration rose from 5.580 in the control to 31.035 at the first introduction but decreased at higher concentration of Cd in the mixture. The literature is scanty on the reports of metal mixture solution and their effects on their bioaccumulation in their parts but from this study, it showed that the presence of a particular metal even at low concentrations can lead to decrease in bioaccumulation of the other metal.
Zinc and cadmium have many physical and chemical similarities as they both belong to group IIB of the periodic table. They are usually found together in the ores and compete with each other for various ligands. Thus interaction between these two metals in the biological system is likely to be similar. The fact that cadmium is a toxic heavy metal and zinc is an essential element makes the association interesting as it raises the possibility that the toxic effects of cadmium may be preventable or treatable by zinc.
Conclusion
To avoid entrance of metals (Cd and Zn) into the food chain, municipal or industrial waste should not be drained into rivers and farmlands without prior treatment. Apart from treating the discharge that enters into the farms, it is also imperative to utilize alternative measures of cleaning up the already contaminated substrates. The daily intake of these metals could however change depending on the dietary pattern of each community and the volume of contaminants added to the ecosystems. Leafy vegetable like C. olitorius has been shown to accumulate relatively high concentrations of these metals compare to fruit vegetables.
References
- Al-Yousef, H.M.; Amina, M.; Ahamad, S.R.; Biomedical Res.2017, 28(10):4581-4587.
- Oyedele, D. J.; Asonugho, C.; Awotoye, O. O.;Electronic J. Environ. Agric. Food Chem.2006;5(4):1446-1453.
- Musa, A.; Ogbadoyi, E.O.;J.Nutrit.Food Sci. 2012;2(3)doi:10.417212155-96000;8pages
- Musa, K. T.; Muktar, L.; Gemma, G. J. H.; Taoffeq R.;Vegetables.2010,2:668.
- Olawuyi, P.O.; Falusi, O.A.; Oluwajobi, A.O.; Azeez, R.A.; Akomolafe, J.F.; Mayaki, H.;Euro. J. Biotech. Biosci.2014;2(1):1-3.
- Fasinmirin, J. T.; Olufayo, A. A. J.Medicinal Plants Res.2009,3(4):186-191.
- Li, OS; Cai,SS; Mo, CH; Chu, B; Rang, LH; Yang, FB; Ecotox. Env. Safety.2010;73:84-88.
- Giwa, A.A.; Amuda, O.S.; Bello, I.A.; Bello, M.O.;Int. J.Phys.Sci. 2007;2(1): 47-52.
- Singh, J.; Upadhay, S.K.; Pathak, R.K.; Gupta, V.; Toxic.Env.Chem.2011;93(3): 462-493.
- Amuda, O.S.; Giwa, A.A.; Bello, I.A.; Biochem.Eng.J.2007;36 :174-181.
- Bigdel, M.; Seilsepour, M; Am. Eura. J.Agric. Environ. Sci.2008;4(1):86-92.
- Lim, C. Y.; Yoo, Y. H.; Sidharthan, M.; Ma, W. M.; Bang, I. C.; Kim, J. M.; Lee, K. S.; Park, N. S.; Shin, H. W.; J. Environ. Biol.2006;27:61-466.
- Chen, X.; Wang, J; Shi, Y.; Zhao, M.O.; Chi, G.Y.;Bot.Stud. 2011;52:41-46.
- Giwa, A.A.; Abdus-Salam, N.; Amuda, O.S.; Bello, O.S.; Int.J.Biosci.2007;2(1):40-47
- Singh, D.; Chhnokar, P. K.; Pandey, R.N.; IARI; New Delhi,1999..
- Rattan, R. K.; Datta, S. P.; Chhonkar, P. K.; Suribabu, K.; Singh, A. K.; Agric. Eco. Environ.2005;109:310-322.
- Wang, X.; Sato, T.; Xing, B.; Tao, S.; Sci. Tot. Env.2005;350:28-37.
- Cheng, SF; Huang, CY.; Int.J.Appl.Sci.Eng.;2006;4(3):243-252.
- Singh, S.; Zacharias, M; Kaipana, S; Mishra, S.; J.Env.Chem.Ecotox.2012;4(4):75-81
- Audu, A.A.; Lawal, A.O.;J.Appl.Sci.Environ.Mgt.2006;10(2):105-109