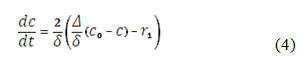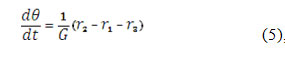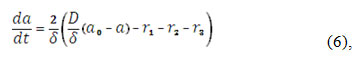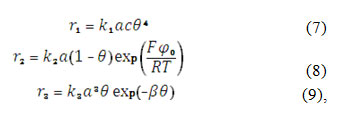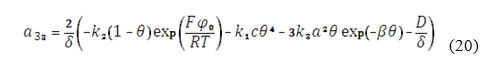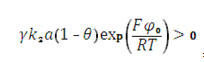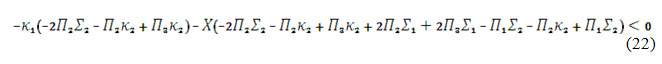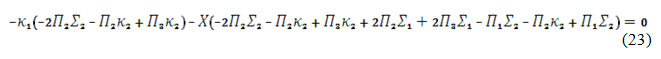Introduction
The use of chemically modified electrodes (CME) is a very important step in electroanalytical chemistry [1 – 10]. Compared to the bare electrodes, they have some advantages, like:
- Rapidity;
- Low cost;
- Precisity;
- Exactity
- Flexibility;
- Versatility in use;
- Affinity between the electrode modifier and the analyte.
On the other hand, not only human, but also zoonotic tuberculosis is an important public health concern worldwide, especially in developing countries [11]. In developed countries, the disease has almost been eradicated after the implementation of preventive and control measures such as testing, culling or pasteurisation of milk, but for developing countries, the tuberculosis combat remains an actual problem.
Isoniazid [12 – 14] is a widely used drug in treatment of tuberculosis – not only in human, but also in mammal organisms (dogs and cats). It is also used to treat actynomicosis in cattle. Its mechanism of action includes the inhibition of synthesis of mycolic acid in Mycobacterium tuberculosis, but includes side effects [15 – 17], like euphoria, digestive diseases and liver disorders, due to hydrazine formation in its metabolism. Moreover, the milk and meat of cattle, treated with isoniazid is also prohibited to use. Thus, the development of a method, capable to determinate its concentration efficiently, is really actual task and the electrochemical methods involving CME, yet used for other compounds [1 – 10], may be an excellent solution to this task [18].
Although, by far, the electrodes, modified by CoO(OH), haven´t been used for hydrazine and its derivatives, this material, yet used for phenolic [19] and oxalate [20] compounds, oxidizing in similar conditions, may have potential electrocatalyzing action for isoniazid. CoO(OH) is seen as an alternative for titanium (IV) oxide in photo- and photoelectrocatalyst systems, cause it is a p-type semiconductor, forming dark-colored films (from dark-brown to black) [21 – 23].
The development of new analytical techniques, involving CME encounters some difficulties, like:
- Indecision in the most probable mechanism for the modifier electroanalytical action;
- The possibility of electrochemical instabilities (oscillatory or monotonic), characteristic for the electrooxidation of different organic and inorganic compounds (including the electropolymerization process) [24 – 32], and capable to give strong impact to analytical signal interpretation;
- The absence of a rigid theoretical base, describing the behavior of the analyte and the modifier.
A mechanistic theoretical investigation, including the development and analysis of a mathematical model, capable to describe the system´s behavior, is an interesting manner to resolve all three mentioned difficulties and to predict the behavior of an electroanalytical system a priori. It also gives the possibility to compare the behavior of this system with that of similar ones without experimental essays.
So, the general aim of this work is the mathematical mechanistic evaluation of the possibility of use of CoO(OH) as an electrode modifier for isoniazid oxidation in lightly alkaline media. It will require the realization of specific objectives: the mechanism suggestion, the development and analysis of the mathematical model, corresponding to the case, and the comparison of its behavior with that of analogous systems, involving CoO(OH) [33 – 35].
System And Its Modeling
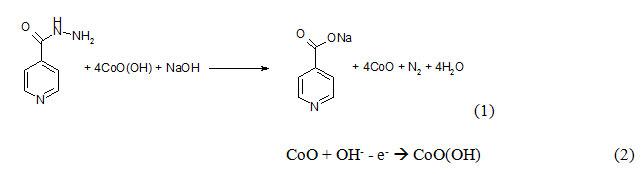
It is known from [18], that the electrooxidation of isoniazid is realized in alkaline solution. On the other hand, from [19 – 23] it is known, that CoO(OH) is also obtained in the same media. So, the mechanism for CoO(OH)-assisted isoniazid electrooxidation may be predicted as:
Contrarily to the cases of [19 – 20], observed in [33 – 34], the hydroxyl ion takes the active part not only in CoO(OH) formation, but also in the analyte oxidation. Moreover, if the media is strongly alkaline, it may destroy the material:
So, to describe the behavior of this system, we use three variables:
c – the isoniazid concentration in the pre-surface layer;
θ – the CoO(OH) coverage degree;
a – the alkali concentration in the pre-surface layer.
To simplify the modeling, we suppose that the reactor is intensively stirred, so we can neglect the convection flow. Also we suppose that the background electrolyte is in excess, so we can neglect the migration flow. The pre-surface layer thickness is assumed to be constant, equal to δ, and the concentration profile of the analyte and the alkali, to be linear.
The analyte enters in the pre-surface layer by its diffusion and reacts with CoO(OH) in the presence of the alkali. Thus, taking in account the first Fick’s law and the double electric layer (DEL)-related modeling coefficient, the balance equation will be rewritten as:
in which r1 is the reaction rate of the reaction (1), c0, the analyte bulk concentration, Δ, its diffusion coefficient.
The CoO(OH) is formed by the reaction (1) from CoO, initially covering the entire electrode surface. It reacts with the analyte (1) and may be destroyed by the reaction with alkali (3). So, its balance equation will be rewritten as:
in which r2 and r3 are reaction rates of the reactions (2) and (3) and G is the CoO(OH) maximal surface concentration.
The alkali enters in the pre-surface layer by its diffusion and enters in the reactions (1), (2), and (3). So, its balance equation will be rewritten as:
in which D is the alkali diffusion coefficient and a0 its bulk concentration. The correspondent reaction rates may be calculated as:
In which the parameters k stand for correspondent rate constants, F is the Faraday number, φ0 is the potential slope in DEL, related to zero-charge potential, R is universal gas constant, T is the absolute temperature and β is the variable, describing the CoO(OH) adsorbed particles’ interaction. The parameter φ0 depends of , due to the surface composition impacts. This dependence may be supposed as linear, described like:
In which the parameter y describes the changes of capacitances and charges in DEL, occurring during the electrochemical reaction.
In general, this model resembles the yet described in [33 – 34]. Nevertheless, as hydroxyl participates directly in the analytical reaction of the analyte, the use of neutral solutions in the analysis will be even more difficult for electroanalysis. The common and different features of this systems and the analogous ones will be discussed below.
Results and Discussion
To investigate the system with the CoO(OH)-assisted isoniazid electrochemical detection, we analyze the equation set (4), (5), (6), taking in account the algebraic relations (7 – 10), by means of linear stability theory. The Jacobian functional matrix steady-state elements may be described as:
Taking in account the expressions (11), 16) and (20), one can see that the positive addendums, describing the positive callback, are present in them. So, the oscillatory behavior, typical for suchlike systems [33 – 35], is possible. It may be caused by surface and electrochemical factors.
if the CoO(OH) electrosynthesis is provided in condicions, in which CoO is strong reducent and in which DEL influences are very strong. It explains the oscillation amplitude dependence on the solution medium;
if the CoO(OH) film dissolution is accompanied by attraction of its adsorbed particles, which leads to the surface instability. Both factors are typical for the systems [33 – 34] in strongly alkaline medium. The oscillations are expected to be frequent and of short amplitude.
The steady-state stability will be investigated by Routh-Hurwitz criterion. In order to apply it for the equation set (4 – 6), avoiding cumbersome expressions, we introduce new variables, for the Jacobian determinant to be described as:
Opening the brackets and applying the Det J<0 inequation, the steady-state stability requirement will be described as:
This inequation will be satisfied, in the case of the negativity of and the positivity of , describing the fragility of DEL influences of electrosynthesis of CoO(OH) and repulsion of its adsorbed particles. The reaction will be diffusion controlled, and the diffusion of analyte will be slower than that of alkali.
The electroanalytical efficiency of steady-state is pH-dependent. In strongly alkaline media, the steady-state will be stable, but the material will be dissolved, and that steady-state would not be efficient. So, the optimal pH for analysis varies from 8 to 12. The pH has to be high anough to maintain the electroanalytical reaction and low enough for the material to be stable. This zone corresponds to linear part of electrochemical parameter – concentration curve, so CoO(OH) may be an efficient electrode modifier for isoniazid analysis.
When the stabilizing and destabilizing influences are equal, the monotonic instability is realized, and its condition is:
It is correspondent to N-shaped part of voltamperogram, and in this point, the stable steady-states have the margin with unstable ones. In the case of the presence of interfering compounds, the model will be more complicated, cause either CoO(OH), or the analyte will be sensitive to their behavior. Some relevant cases of that presence will be described in our next works.
Conclusions
From the mechanistic analysis of the possibility of CoO(OH)-assisted isoniazid electrochemical detection, it is possible to conclude that:
- CoO(OH) may be an excellent electrode modifier for isoniazid, in lightly and moderately alkaline solutions. Highly alkaline solutions may lead to the material destruction, and the steady-state stability won’t be electroanalytically efficient;
- the steady-state stability is warranted by the absence of strong DEL influences of CoO(OH) formation and CoO(OH) particles’ repulsion during its dissolution. In the mentioned condition, the reaction is diffusion-controlled;
- the oscillatory behavior in this system is possible, being caused by surface and electrochemical factors. The time dissipative structure’s existence will be maintained by analyte and alkali diffusion and by [Co(OH)6]3- complex formation.
References
- Beitollahi, H. Karimi-Maleh, I. Sheikhoae, Casp. J. Chem. 1:17; 2012.
- H. de Oliveira, A.C. Dias Souza, L. Pizzuti, et. al., Orbital. Elec. J. Chem. 6:255; 2014.
- Scarpetta, A. Mariño, K. Bolaños et. al., Rev. Colomb. Cien. Quím. Farm. 44:311; 2015.
- Lin, RSC Adv. 5: 9848; 2015.
- Li, X. Li, Y.Zhang et. al., RSC Adv., 5:5432; 2015.
- Q. Huang, Ch. Chen, Y.-M. Wu et. al., Int. J. Electrochem. Sci. 7:5510;2012.
- B. Raoof, A, Kiani, R. Ojani, R. Valliolahi, Anal. Bioanal. Electrochem. 3:59;2011.
- Khajvand, R. Ojani, J.-B. Raoof, Anal. Bioanal. Electrochem. 6:501;2014.
- Ojani, V. Rahimi, J. Raoof, J. Chin. Chem. Soc. 62:90;2015.
- R. Mantesha, B.E. Kumara Swamy, K. Vasantakumar Pai, Anal. Bioanal. Electrochem. 6:234;2014.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2710499/#R37, accessed at 2nd of January 2017.
- https://www.drugs.com/cdi/isoniazid.html, accessed at 2nd of January 2017.
- T. Ayele, M.S.M. van Mourik, T. P. A. Debray, M. J. M. Bonten, PloS One, 10;2015, e0142290, DOI: 10.1371/journal.pone.0142290
- S. Dean, S.G. Rhodes, M. Coad et. al., Tuberculosis. 88:586;2008.
- L. Goldman, S. S. Braman, Chest. 62:71;1972.
- https://pubchem.ncbi.nlm.nih.gov/compound/isoniazid#section=Health-Hazard, accessed at 2nd of January 2017.
- Abdo Arpex, M. C. Lima Varella, H. Ribeiro de Siqueira, F. A. Fiúza de Mello, J. Braz. Pneumol. 36:626;2010.
- http://emedicine.medscape.com/article/180554-overview, accessed at 2nd of January 2017.
- Karim-Nezhad, S. Pashazadeh, Iran. J. Anal. Chem. 2:100:2015.
- Stadnik, E.M. Caldas, A. Galli, F.J. Anaissi, Orbital. Elec. J. Chem. 7:122;2015.
- S. Bonini, F.Q. Mariani, E. Guimarães Castro et. al., Orbital Elec. J. Chem. 7:318;2015.
- W. Wang, Yi Ming Kuo, Phys. Stat. Sol. 210:494;2013.
- D. Jagadale, D.P. Dubal, C.D. Lokhande, Mat. Res. Bull. 47:672;2012.
- Yang, H. Liu, W.N. Martens, R.L. Frost, J. Phys. Chem. C. 114:111;2010.
- Hudson, M.R. Bassett, Rev. Chem. Eng. 7:108;1991.
- Pagitsas, S. Dimitra, Electrochimica Acta. 36:1301;1991.
- J. Pearlstein, J.A. Johnson, J. Electrochem. Soc. 136:1290;1991.
- Das, N.R.Agrawal, S.A.Ansari, S.K.Gupta, Ind. J. Chem. 47:1798;2008.
- U. Rahman, M.S. Ba-Shammakh, Synth. Met. 140:207;2004.
- S.Liu, M.A.S. Oliveira. J. Braz. Chem Soc. 18:143;2007.
- Sazou D., Met. 130:45;2002.
- Das, N. Goel, N.R. Agrawal, S. K. Gupta, J. Phys. Chem. 114:12888;2010.
- Bazzaoui, E.A. Bazzaoui, L. Martins, J.I. Martins, Synth. Met. 130:73;2002.
- Tkach, S.C. de Oliveira, G. Maia et. al., Mor. J. Chem. 4:112;2016.
- Tkach, S.C. de Oliveira, F.J. Anaissi et. al., Anal. Bioanal. Electrochem. 8:1;2016.
- V. Tkach, V. Nechyporuk, O. Slipenyuk, Eclet. Quím. 37:74;2012.