Introduction
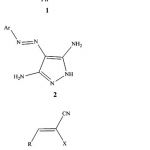
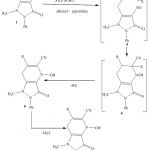
The present work describes the synthesis of several new pyrazolo[3,4-b]pyridines and pyrazolo [1,5-a]pyrimidine derivatives as potential schistosomocidal agents from 1,5-dimethyl-4-nitroso-2-phenyl-1H-pyrazol-3(2H)-one 1 and 4-(aryldiazenyl)-1H-pyrazole-3,5-diamines 2 as starting components .Thus,it has been found that , 1,5-dimethyl-4-nitroso-2-phenyl-1H-pyrazol-3(2H)-one 1 reacted readily with the arylmethylenemalononitriles 3b,c in ethanol containing catalytic amount of piperidine to yield products via hydrogen cyanide and water elimination .The pyrazolo[3,4-b]pyridine structures7 were assigned as reaction products. IR spectra of 7 showed absorption bands corresponding to the cyano groups and the the carbonyl functions of antipyrinyl moiety ,and clearly indicates the absence of the amino functions.The same products 7 were obtained by reacting 1,5-dimethyl-4-nitroso-2-phenyl-1H-pyrazol-3(2H)-one 1 with ethyl α-cyanocinnamates 3e,f via formal elimination of one mole carbon monoxide ,ethanol and water.Compound 7 were proposed to be formed through addition of the methyl group in 1 to the activated double bond in 3 to give the adducts 4 which cyclized to 5 followed by losing one mole of hydrogen cyanide or ethyl formate to afford the intermediate 6,which aromatized into 7 via water elimination (cf.Scheme 1).
 |
 |
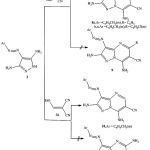
Also,we have found that, 4-(aryldiazenyl)-1H-pyrazole-3,5-diamines 2 reacted with arylmethylenemalononitriles 3a,b in ethanol / piperidine for which several isomeric structures seemed possible.Acyclic structures were readily eliminated based on IR spectra which revealed only one cyano band.Also,7-aminopyrazolo [1,5-a]pyrimidines 8 were preferred over isomeric 5-aminopyrazolo [1,5-a]pyrimidines 9 based on 1H-NMR spectrum which revealed an amino protons at δ > 7.0ppm.if these products were the isomeric 9 ,the amino signal may be observed at δ ≈ 4-6ppm.Deshielding effect of the pyrazolopyrimidine-7-amine protons by ring nitrogen anisotropy has been previously described 4,5. Structures 8 were suggested to be formed by initial addition of the exocyclic amino function of 2 to the activated double bond in 3 ,followed by cyclization through addition of the endocyclic pyrazole nitrogen to the cyano group to give 7aminopyrazolo [1,5-a]pyrimidines 8.
In addition, 4-(aryldiazenyl)-1H-pyrazole-3,5-diamines 2 cyclocondensed with ethoxymethylenemalononitrile 3d in ethanol containing catalytic amount of piperidine to yield product of condensation by elimination of ethanol and hydrogen.this product may be formulated as 7-aminopyrazolo [1,5-a]pyrimidines 10 or 5-aminopyrazolo [1,5-a]pyrimidines 11.Structure 10 was preferred over possible 11based on 1H-NMR spectrum which showed amino protons at δ ≈ 7.0ppm 4,5.If this product was 11,amino protons at much higher field would be appeared at δ ≈ 4-6ppm4,5. Formation of compound 10 was proposed to proceed via condensation of the exocyclic amino group in 2 with the ehtoxy group in 3d followed by addition of the pyrazole NH to the cyano goup to form 7-aminopyrazolo [1,5-a]pyrimidines 10 (cf.Scheme 2).
 |
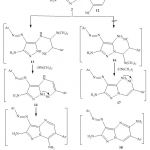
Enaminonitriles constitute an interesting class of compounds that are versatile reagents for the synthesis of different heterocycles 2,6-8.Thus,in continuation to this interest,we report here the reactivity of 2 towards 2-(1H-benzo[d]imidazol-2-yl)-3-(dimethylamino)acrylonitrile 12. Therefore,compound 2 reacted with c in refluxing ethanol and in presence of few drops of acetic acid to yield product for which 7-aminopyrazolo [1,5-a]pyrimidines 15 or 5-aminopyrazolo [1,5-a]pyrimidines 18 can be formulated. However,structure 15 was inferred from its 1H-NMR spectrum .Although the endocyclic imino group is the most nucleophilic centre ,nevertheless,it is the most sterically hindered site.Compound 15 was proposed to be formed by first addition of the exocyclic amino group in 2 to the double bond in 12 followed by elimination of dimethylamine to give non-isolable acyclic intermediate 14 ,which readily undergoes interamolecular cyclization by addition of the pyrazole NH to the cyano group to yield 7-aminopyrazolo [1,5-a]pyrimidines 15 (cf.Scheme 3).
 |
Experimental
All melting points are uncorrected and measured on Griffin & George MBF 010T (London) apparatus. Recorded yields correspond to the pure products. IR (KBr) spectra were recorded on a perkin Elmer SP-880 spectrophotometer and 1H-NMR spectra were measured on a Varian 300 MHz at Cairo University spectrometer in DMSO-d6 as solvent and TMS as an internal standard (Chemical shifts are reported in δ units ppm). Mass spectra were measured on GS/MS INCOL XL Finnegan MAT. Microanalysis were performed on LECOCHN-932 and carried out in the Micro analytical Data Unit, Cairo University.
Formation of 6-aryl-1-methyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazolo[4,3-b] pyridine-5-carbonitriles(7a,b)
Method A:
A solution of 1,5-dimethyl-4-nitroso-2-phenyl-1H-pyrazol-3(2H)-one 1 (0.01mole) and arylmethylenemalononitriles 3a,b (0.01mole) in ethanol (50ml) containing catalytic amount of piperidine (0.1ml), was heated under reflux for three hours then left to cool.The solids formed were collected by filtration , recrystallized from the suitable solvents and then identified as 7a,b.
Method B:
A mixture of 1 (0.01mole) and arylmethylenemalononitriles 3e,f (0.01mole) in ethanol (50ml) were refluxed for six hours in presenc of (0.1ml) of piperidine and the solvent was concentrated to its half volume and then left to cool.The solids deposited were filtered off, recrystallized then identified (m.p.,mixed m.p.and i.r.) as 7a,b.
6-(3-Chlorophenyl)-1-methyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazolo[4,3-b]pyridine-5-carbonitrile (7a)
Yellow crystals from ethanol,m.p.250-2520C,yield 65%.IR(υmax / cm-1):2228(conjugated CN), 1669(CO). 1H-NMR (DMSO-d6): (δ pm):3.30(s,3H,N-CH3),7.30-7.70 (m,10H,aromatic protons).C20H13ClN4O)(360.80) : Calcd.C,66.58; H,3.63; N, 15.52%,Found : C,66.68; H,3.71; N, 15.32%.
1-Methyl-6-(4-nitro-1H-pyrrol-2-yl)-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazolo[4,3-b]pyridine-5-carbonitrile (7b)
Faint brown powder from ethanol / dimethylformamide ,m.p.> 3000C,yield 70%.IR(υmax / cm-1) :2227 (conjugated CN), 1675(CO).C18H12N6O3)(360.33) : Calcd. C, 60.00; H, 3.36; N, 23.32%,Found : C,66.08; H,3.51; N, 23.37%.
Preparation of 2,7-diamino-5-aryl-3-(aryldiazenyl)pyrazolo [1,5-a]pyrimidine-6-carbonitrile (8a,b)
A solution of 4-(aryldiazenyl)-1H-pyrazole-3,5-diamines 2 (0.01 mole) and arylmethylenemalononitriles 3a,b (0.01mole) in ethanol (50 ml) were refluxed for three hours in presenc of (0.1ml) of piperidine and then left to cool.The resulting solid products were filtered off, recrystallized from the suitable solvents and then identified as 8a,b.
2,7-Diamino-5-phenyl-3-(m-tolyldiazenyl)pyrazolo[1,5-a] pyrimidine-6-carbonitrile (8a)
Yellow crystals from methanol, m.p.288-2900C,yield 65%.IR(υmax / cm-1):3604,3572,3470(NH2),2215(conjugated CN), 1605(-N=N-). 1H-NMR (DMSO-d6): (δ pm):2.35(s,3H,CH3) , 7.22 (s,1H,NH2), 7.34-7.79 (m,9H,aromatic protons) ,8.61(s,2H,NH2) . C20H16N8)(368.40) : Calcd.C,65.21; H,4.38; N, 30.42. %,Found : C, 65.33; H,4.42; N, 30.35%.
2,7-Diamino-5-(4-chlorophenyl)-3-(m-tolyldiazenyl)pyrazolo [1,5-a]pyrimidine-6-carbonitrile (8b)
Brown crystals from methanol, m.p.288-2900C,yield 65%.IR(υmax / cm-1):3580,3460,3302 (NH2),2209 (conjugated CN), 1615(-N=N-). C20H15ClN8) (402.85) : Calcd.C,59.63; H,3.75; N, 27.82. %,Found : C, 59.51; H,3.67; N, 27.68%.
2,7-diamino-3-(m-tolyldiazenyl)pyrazolo[1,5-a]pyrimidine-6-carbonitrile (10)
A solution of 4-(aryldiazenyl)-1H-pyrazole-3,5-diamines 2 (0.01mole) in ethanol (50ml) was treated with (0.01mole) of ethoxymethylenemalononitrile 1d and two drops of piperidine were refluxed for three hours and then left to cool at room temperature .The precipitate separated on cooling was filtered off, recrystallized from ethanol to give 10 as yellow crystals, ,m.p.> 3000C,yield 70%. IR(υmax / cm-1):3422,3273,32000(NH2),2215(conjugated CN), 1605(-N=N-). 1H-NMR (DMSO-d6): (δ pm):2.30(s,3H,CH3) , 7.21(s,2H NH2),7.15-7.60 (m,3H,aromatic protons),7.96(s,1H, aromatic proton) ,8.42(s, 1H, pyrimidine ,H-5),8.63(s,2H NH2) . C14H12N8)(292.31) : Calcd.C,57.53; H,4.14; N, 38.33. %,Found : C, 57.23; H,4.70; N, 38.30%.
Synthesis of 3-(aryldiazenyl)-6-(1H-benzo[d]imidazol-2-yl) pyrazolo [1,5-a] pyrimidine-2,7-diamine (15a,b)
A mixture of 4-(aryldiazenyl)-1H-pyrazole-3,5-diamines 2 (0.01mole ) and (0.01mole) of 2-(1H-benzo[d]imidazol-2-yl) -3-(dimethylamino) acrylonitrile 12 in ethanol (50ml) containing acetic acid (1ml) was refluxed for one hour.The deposited solid was collected by filtration and recrystallized then identified as 15a,b.
6-(1H-benzo[d]imidazol-2-yl)-3-((4-chlorophenyl)diazenyl) pyrazolo[1,5-a]pyrimidine-2,7-diamine (15a)
Yellow crystals from acetic acid , m.p.> 3000C,yield 75%. IR(υmax / cm-1) :3480,3410,3316 (NH2), 1605(-N=N-). 1H-NMR (DMSO-d6): (δ pm): 7.33(s,2H NH2) ,7. 50-8.08 (m,8H,aromatic protons), 8.59(s, 1H, pyrimidine ,H-5),8.99(s,2H NH2),9.75(s,1H,NH) . C19H14ClN9) (403.83) : Calcd.C,65.51; H,3.49; N, 31.22. %, Found : C, 65.50; H,3.80; N, 31.34%.
6-(1H-benzo[d]imidazol-2-yl)-3-(m-tolyldiazenyl)pyrazolo[1,5-a]pyrimidine-2,7-diamine (15b)
Brown powder from ethanol / dimethylformamide , m.p.261-263 0C,yield 70%. IR(υmax / cm-1) :3418, 3356,3273 (NH2), 1610(-N=N-). 1H-NMR (DMSO-d6): (δ pm): 2.20(s,3H,CH3),7.35(s,2H NH2) ,7. 47-8.11 (m,7H,aromatic protons), 8.61(s, 1H, pyrimidine ,H-5),8.90(s,2H NH2),9.72(s,1H,NH) . C20H17N9) (383.42) : Calcd.C,62.65; H,4.47; N, 32.88. %, Found : C, 62.45; H,4.36; N, 32.79%.
References
- M.A.Sofan,F.M.A.El-Taweel,T.M.AbuEl-Maati and A.A.Elagamey, Pharmazie. 49;482;1994.
- A.El-Nezi,B.Al-saleh and M.H.Elnagdi, J.Chem.Res. (S)4 ,(M) 116;1997.
- A.A.Elagamey and F.M.A.El-Taweel, J. Prakt.Chem. 333,333;1991.
- M.H.Elnagdi,K.Y.Sadek F.M.abdelGalil and S.M.E.Hassan, Arch. Pharm. 321,851;1988.
- M.H.Elnagdi and A.W.Erian, Bull.Chem.Soc.Jpn. 63,1854;1990.
- K.M.al-Zayadi and E.A.Hafez, J.Chem.Res.(S)360 ,(M) 1621;1999.
- R.lue and J.V.Greenhill, Adv.Heterocycl.Chem. 67,209;1994.
- U.Kuklander,Enaminones as Synthons ,In the Chemistry of Enaminones ,ed.,Z.Rappoport,Wiley,New York,London,Sydney, Toronto. 523-636;1994.
