Introduction
Vanadium is a trace element of highly critical role in biochemical processes and of significant importance in environmental, biological and industrial analysis due to its toxicity. Vanadium is an essential oxygen carrier for marine organism [1,2]. Industrial exposure of vanadium has shown
to cause eye and lung irritation due to inhibition of activity of enzyme cholinesterase [3,4]. It is mainly found in teeth, bone, hairs and nails [5]. However, it becomes toxic when its concentration exceeds 0.5 ppm. The toxicity of vanadium is dependent on its oxidation state, with vanadium(V) being more toxic than vanadium(IV) [6].FAAS with its relative low cost and decent analytical performance is the important instrument in the research laboratories for determination of a range of heavy metals. Accurate determination of trace heavy metals by FAAS is usually one of the important problems for the analytical chemist because of their low concentrations. Likewise other important problem in FAAS determinations of heavy metals are the effects of the matrix of the analyzed samples. In order to overcome, these problem analytical chemists mostly use separation and preconcentration methods such as coprecipitation, liquid–liquid extraction (LLE), cloud point extraction (CPE), electrodeposition, SPE, solid phase micro extraction (SPME), etc. [3-5] .
In recent years, SPE method has been well used for determination of Vanadium ions in numerous environmental samples [9, 7] because of its simplicity, rapidity, minimal cost, low consumption of reagents and its ability to combine with different detection techniques either in on-line or off-line mode [8]. The main part of the SPE is the sorbent material that determines the selectivity and sensitivity of the technique. However, the commonly used SPE sorbents, such as C18 silica and graphitic carbon, are often the only suitable option for a limited number of analytes. Reusability of the SPE column is also a problem. Thus, developing new SPE sorbent material is important [9]. Carbon derivatess are well known for their high adsorption capacity. They have been established to possess excessive potential as adsorbents for removing many types of environmental pollutants such as heavy metals[10, 11]: carbon nanotubes (CNTs)[12, 13], Fullerenes can be used as chromatographic stationary phases to offer high selectivity for specific compounds[14, 15] or as sorbent materials for on-line clear up and preconcentration [16, 17].
Recently, CNTs have been excellent classes of sorbent materials for SPE [18]. Since the first application of CNTs in SPE by Cai et al. that he used multi-walled carbon nanotubes (MWCNTs) [18], in recent years many reports have been distributed focusing on progress of use CNTs-based SPE methods for a great variety of analytes, including phenolic compounds [19], insecticides [20], pharmaceuticals [21, 22], inorganic ions [23], organometallic compounds [24]. Then, the selectivity of adsorbent (CNTs) can be controlled by covalently or non-covalently modification of the CNTs functional groups. In addition, inherent properties of CNTs such as well chemical and thermal stability make them appropriate to be used as adsorbents for SPE column. Other carbon allotropes, for instance, graphite and diamond, have also been established as adsorbents in SPE or SPME [25].
Nevertheless, the sorbents have the advantages of no swelling, fast kinetics, and good mechanical stability as well [22]. Recent research on the use of surfactant-coated mineral oxides for SPE have demonstrated these new sorbent materials to be a promising tool for the pre-concentration of organic compounds in a wide polarity range [23]. The most prominent among the supports used are silica gel, [24, 25-28] SDS coated alumina, [29] and modified chromosorb and C18 bonded silica [30,35-39].
In this study, we report the synthesis of this new sorbent and its application as a selective sorbent for separation, preconcentration and determination of Vanadium(V) ions octafunctionalized calix[4]resorcinarene-hydroxamic acid(OFCHA) (Schematic 1.)by FAAS determination.
Materials and Methods
Apparatus
The concentration of the metal ion solutions was determined by using the Varian model spectra AA-240 (Mulgrara, Victoria, Australia). The pH-measurements of the metal ion and buffer solutions were carried out by an Orion 420. Infrared spectra of PANF were carried out from KBr by a Perkin-Elmer 1430 ratio recording spectrophotometer.
Materials and reagents
All the necessary materials and reagents were of analytical grade and were purchased Merck, Aldrich and Sigma Company. All the dilutions were prepared by ultrapure deionized water. The octafunctionalized calix[4]resorcinarene-hydroxamic acid (OFCHA) was synthesized and characterized as reported earlier [31].
![Schematic 1. Structure of octa-functionalised calix[4]resorcinarene-hydroxamic acid (OFCHA).](http://unitedjchem.org/wp-content/uploads/2018/12/Vol_1No_2_Fab_Ali_Sch1-150x150.jpg) |
Scheme 1: Structure of octa-functionalised calix[4]resorcinarene-hydroxamic acid (OFCHA). |
Preparation of Modified nano polyacrylonitrile fiber
Modified nano polyacrylonitrile fiber was prepared by adding 3 g of acrylic fibers to 300 ml of Calix[4]resorcinarene-Hydroxamic Acid(OFCHA) with different concentration solutions. Electro Spinning is done in according to reference[33]. The adsorption ions onto PANF-(OFCHA)for Vanadium (V) ions were investigated using the batch method [28].
Column preparation and recommended procedures
The adsorption of Vanadium by the adsorbents was studied by the SPE technique. The SPE mode adsorption was selected because of its simplicity, fast and economic for the preconcentration and determination of trace amounts of Vanadium in different samples. PANF–(OFCHA) (0.1 g) was placed in a 2 mL SPE column using an upper frit and a lower frit to avoid adsorbent loss. Prior to extraction, the column was conditioned with 10mL of MeOH and 10mL of deionized water, respectively. The sample solution (50mL) was passed through the column at a flow rate of 2mL/min. Then, the column was washed with 5mL of 10% (v/v) MeOH aqueous solution to remove the co-adsorbed matrix materials from the column. The analytes retained on the column were eluted with 5mL of 4M HNO3 aqueous solution that flow rate of eluent was 2ml/min.
Finally, the analyte ions in the eluent were determined by FAAS at 228.8 nm for Vanadium. The effects of several parameters, such effect of pH, eluent type and its volume, effect of flow rates of sample and eluent solution and amount of adsorbent, effect of interfering ions and breakthrough volume were also studied. The results of these studies were used to obtain the optimum conditions for adsorption capacity measurements. Using the procedure described above, the percent of recovery was calculated from the following equation) Eq. (1):
Where Cs is analyte concentration in eluent (found by FAAS) (mg/L), and Cw is analyte concentration in sample solution (known) (mg/L), Vs is volume of eluent (mL) and Vw is volume of sample solution (mL).
Sampling
Tap water(Tehran, taken after 10 min operation of the tap), rain water (Tehran, 20 January, 2015), and Sea water (taken from Caspian sea, near the Mahmoud-Abad shore) samples were analysed (Table 2).Tap water samples used for development of the method were collected in glasses containers. Before the analysis, the organic content of the water samples was oxidized in the presence of 7% H2O2 and then concentrated nitric acid was added. These water samples were then filtered through a 0.45µm Millipore cellulose membrane to remove suspended particulate matter and stored in a refrigerator at 4 ◦C in the dark before analysis.
Results and Discussion
Some preliminary experiments were carried out in order to investigate the extraction of Vanadium by the PANF–(OFCHA)from solution. The results showed that PANF-(OFCHA)could extract it quantitatively. Figure 1 shows SEM image of TEM images of the nano polyacrylonitrile fiber.
 |
Figure 1: SEM image of TEM images of the nano polyacrylonitrile fiber |
SEM Investigations
SEM was to examine the external surface of the fiber before and after the modification. As can be seen from Figure 2, acrylic fiber similarly surface (Figure 2 (a)), and with modified fiber (PANF-(OFCHA), obvious change comparing to that of the RAF fiber was observed (Figure 2 (b)).
It is clear that the changes have occurred in the morphology of the fiber but photographs demonstrated that the surface of PANF-(OFCHA)was approximately as smooth, swollen and homogeneous as that of the raw fiber. This can be related to new functional groups that were bigger than (CN) groups.
SEM was examining the morphology of the nano fiber before and after the modified. As can be seen from Figure 3, original acrylic nano fiber comparatively morphology (Figure 3(a)), and with modification nano fiber (PAN-(OFCHA), obvious change compared to that of the unmodified fiber was observed (Figure 3 (b)). The PAN-(OFCHA)was roundelay as that of unmodified fiber acrylic nano fiber. This can be related to the modified treatment and incorporation of new functional groups into the fiber structure.
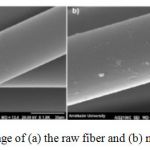 |
Figure 2: SEM image of (a) the raw fiber and (b) modified PAN fiber |
 |
Figure 3: SEM image of (a) (c) the raw nano fiber and (b)(d) modified PAN nano fiber |
Optimization of SPE procedures
Effect of pH
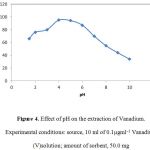
The metal chelate stability constant and its chemical stability considerably influence the SPE recovery. The pH plays a very important role on metalchelate formation and following extraction. Therefore, pH was the first optimized parameter. pH of the analyte solutions was adjusted to desired values with diluted hydrochloric acid (0.1 mol L−1) and/or ammonia solution (0.1 mol L−1) The variation in recovery of Vanadium(V) with pH is shown in Figure 4. According to the results shown in Figure 4 up to pH 4-4.5, complete recoveries are obtained. However, at higher pH values, percentage recovery decreases. This is due to fact that in an acidic solution the protonation of PANF-CALIX[4]RESORCINARENE-HYDROXAMIC ACID occurs and there is a weak tendency for retention between Vanadium (V) and PANF-CALIX[4]RESORCINARENE-HYDROXAMIC ACID, whereas at higher values (pH>5), Vanadium (V) reacts with hydroxide ions to produce Vanadium (OH)2. Accordingly, pH 4.0 was selected for subsequent work and real sample analysis.
 |
Effect of flow rates of sample and eluent solution
The effects of flow rates of sample solution and eluent solution on the recovery of Vanadium (V) was examined between under the optimum conditions in the range of 1–10.0 mL min−1 by controlling the flow rate with peristaltic pump. The recovery of the ions were independent of flow rate in the range of 0.5–2.0 mL min−1 for eluent solution and range of 1–5.0 mL min−1 for sample solution.
Effect of sample solution volume
Another parameter studied to find the best experimental conditions is the volume of sample solution and/or analyte concentration. For this purpose, 50.0–1100.0 mL of sample solutions containing 5 ppm Vanadium (V) was processed according to the suggested procedure. The recovery of Vanadium(V) was quantitative (>96%) obtained up to a sample volume of 1000.0 mL and the adsorbed Vanadium(II) can be eluted with 5 mL eluent. Therefore, an enrichment factor of 200 was achieved by this technique. Finally In our suggested procedure, a sample volume of 50.0 mL was chosen for preconcentration method.
Effect of amount of sorbent
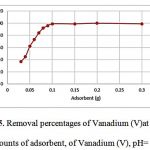
To achieve a high extraction recovery, different amounts of PANF-CALIX[4]RESORCINARENE-HYDROXAMIC ACID ranging from 50 to 300 mg were applied to extract the target compounds from the sample solutions. The results are shown in Figure 5 from which it can be seen that the extraction recovery achieved by 100 mg, but almost the same as obtained with 300 mg or more than of the adsorbent. Based on the above results, 100 mg of PANF-CALIX[4]RESORCINARENE-HYDROXAMIC ACID was selected for the following experiments.
 |
Eluent type and its volume
Other important factors, which affect the percent of recovery, are the type, volume, and concentration of the eluent solution used for the removal of metal ions from the sorbent. For this purpose, various types of eluents were examined according to the suggested procedure. Four mol L−1 HNO3 was found to be adequate for quantitative elution (≥95%). The results show the best quantitative recovery is 5.0 mL of 4.0 mol L−1HNO3 . As a result, 5.0 mL of 4.0 mol L−1 HNO3 was selected in the subsequent preconcentration method.
Table 1: Separation of Vanadium from binary mixtures a
| Diverse ion | Amounts taken(mg) | % Found | %Recovery of Vanadium(V)ion |
| Na+ | 92.4 | 1.15(2.4)b | 98.5(2.7) |
| K+ | 92.5 | 1.36(2.3) | 98.0(2.2) |
| Mg2+ | 24.5 | 0.70(2.6) | 98.5(1.7) |
| Ca2+ | 26.3 | 2.65(3.0) | 98.5(1.8) |
| Sr2+ | 2.45 | 2.85(2.1) | 98.4(2.0) |
| Ba2+ | 3.66 | 3.16(2.1) | 98.7(2.3) |
| Mn2+ | 2.66 | 1.75(2.2) | 96.3(2.3) |
| Co2+ | 2.17 | 6.44(2.3) | 93.0(1.9) |
| Cu2+ | 1.64 | 2.43(2.4) | 93.7(2.4) |
| Zn2+ | 2.76 | 4.97(2.1) | 97.6(2.4) |
| Cd2+ | 2.77 | 2.96(2.4) | 97.2(2.7) |
| Hg2+ | 1.67 | 2.71(2.1) | 97.7(2.7) |
| Ag+ | 2.6i | 3.47(2.9) | 97.6(2.3) |
| UO2+ | 2.76 | 2.74(2.1) | 98.3(2.7) |
aInitial samples contained 10µg Vanadium (V) and different amounts of various ions in 100 mL water.
bValues in parentheses are RSDs based on five individual replicate analysis.
Reusability of Column
The stability and potential regeneration of the column were studied. After every time extraction, the column was washed with 10 mL of MeOH and 10mL of deionized water. Thus, the column was available for a next extraction immediately at least 50 adsorption elution cycles without significant decrease in the recovery of Vanadium (V) ions.
Effect of foreign ions
The influence of common foreign ions on the adsorption of Vanadium (V) on PANF-CALIX[4]RESORCINARENE-HYDROXAMIC ACID was studied. In these work, 50.0 ml solutions containing 5ppm of Vanadium and various amounts of interfering ions were treated according to the suggested procedure. The tolerance level was defined as the maximum concentration of the foreign ion causing a change in the analytical signal no higher than 5%, when compared with the signal of 5-ppm Vanadium alone.
The results, listed in Table 1, demonstrate that the presence of major cations and anions in natural water has no important influence on the adsorption of Vanadium (V) ions under the designated conditions.
Analytical figures of merits
Under optimized conditions, a calibration curve for Vanadium (V)was found by preconcentrating a series of Vanadium (V) standards according to the procedure mentioned. The curve was linear from 1.0 mg/l to 7.0 mg/l for Vanadium.
Table 2: Recovery of Vanadium (V) added to 100mL of different water samples (contaning 0.1M buffer acetic acid / acetate at pH= 4.0).
| Sample | Vanadium(V) spiked | Vanadium(V) detected | Recovery (%) |
| (ng ml−1) | (ng ml−1) | ||
| Sample 1a | 5.0 | 4.6 (2.0)b | 97.7 |
| 10.0 | 9.8 (2.5) | 98.4 | |
| Sample 2c | 5.0 | 4.7 (3.0) | 96.3 |
| 10.0 | 9.6 (3.0) | 97.2 | |
| Tap waterd | 0.0 | 2.5(2.4) | 95.5 |
| 5.0 | 4.5 (2.9) | 97.5 | |
| 10.0 | 15.3 (2.4) | 95.3 | |
| Rain waterf | 0.0 | N.D. | — |
| 5.0 | 4.9 (2.3) | 96.4 | |
| 10.0 | 10.1 (2.6) | 96.6 | |
| Sea waterg | 0.0 | 14.0(2.0) | 97.3 |
| 5.0 | 18.9 (2.3) | 95 .9 | |
| 10.0 | 24.0 (3.0) | 97.9 |
aHg2+, Co2+, Fe3+, Ni2+, Cr3+ , 5000 ng ml−1 of each cation; K+ and Li+, 10,000 ng ml−1 of each
bR.S.D of three replicate experiments.
cHg2+, Co2+,Fe3+,Ni2+,Cr3+ , 2500 ng ml−1 of each cation; K+ and Li+, 5000 ng ml−1 of each.
dFrom drinking water system of Tehran.
eNot detected.
fTehran, 20 january, 2015, Iran
gCaspian sea water.
Table 3: Comparison of some methods for preconcentration and determination of thal-lium with proposed method
| Method | DL (ng ml−1 ) | LDR (ng ml−1 ) | Reference |
| Solid–liquid extraction | 1 | 3.75–17.5 | [30] |
| Potentiometric
stripping |
0.08
1 |
0.1–100
5–250 |
[31]
[32] |
| Liquid–liquid extraction Microcrystalline naphthalene
Proposed method |
4
20 0.167 |
5–20
40–180.0 5.0–240.0 |
[33]
[34] – |
Determination of Vanadium in real water samples
Three type of water samples (information described in section 2.6 sampling) were used for the determination of Vanadium. The analytical results are given in Table 2. The percent of recoveries for the addition of different concentrations of Vanadium (V) to water samples were 97 and 98.9%.These satisfactory percent of recoveries indicate no significant effects from the matrix composition of the real water samples.
Comparison with other solid phase adsorbents
The proposed methodology was compared to a variety of solid adsorbents reported recently in the literature. The distinct features are summarized in Table 3. As can be seen from the table, it is evident that the preconcentration factor obtained with alumina is comparable to or even better than most of the other chelating matrices. The other significant feature of the proposed method is the use of environmentally.
Conclusion
The proposed SPE possesses the following advantages: the technique is rapid when compared with the previously reported procedures for the separation and determination of Vanadium; the time taken for the separation and determination of Vanadium in a 500 mL sample is at the most 30 min. In conclusion, the proposed method reveals the great potential of PANF-CALIX[4]RESORCINARENE-HYDROXAMIC ACID as an advantageous sorbent material in SPE. Using Vanadium(V) as model analyte, the PANF-CALIX[4]RESORCINARENE-HYDROXAMIC ACID packed SPE columns showed reliable and attractive analytical performance in the analysis of environmental water samples. Higher recoveries were achieved with PANF-CALIX[4]RESORCINARENE-HYDROXAMIC ACID than with other adsorbents including C18 silica, graphitic carbon, and CNTs, owing to the large surface area and unique chemical structure of PANF-(OFCHA). Some other advantages of PANF-(OFCHA) as an SPE adsorbent have also been demonstrated, such as high sorption capacity, good reusability, and fine reproducibility. Although the obtained results of this research were related to the Vanadium(V) determination, the system could be a considerable potential guide for the preconcentration and determination of other metals.
Acknowledgment
The authors are so thankful to the Laboratory Complex of I.A.U. for valuable technical assistance and support. The authors declare that there is no conflict of interests.
References
- D.C. Crans, J.J. Smee, E. Gaidamauskas, L. Yang,Chem. Rev. 104:849. 2004.
- F.H. Nielsen, E.O. Uthus, The Essentiality and Metabolism of Vanadium N.D. Chasteen, Kluwar Academic, Dordrecht, 1990.
- W. Plass, Coord. Chem. Rev. 237:205. 2003
- I. Bertni, H.B. Gray, S.J. Lippard, J.S. Valentine, Bioinoganic Chemistry, Viva Books Private Limited, New Delhi. 1998.
- E.K. Paleologos, D.L. Giokas, S.M. Tzouwara- Karayanni, M.I. Karayannis, Anal. Chem. 74:100. 2002.
- B. Patel, G.E. Henderson, S.J. Haswell, R. Grzeskowiak, Analyst 115:1063. 1990.
- Moghimi A. Preconcentration and Determination of Fe(III) Using Octadecyl Silica Membrane Disks and Flame Atomic Absorption Spectrometry, Oriental Journal of Chemistry. 22(3),527-535. 2006.
- Moghimi A., Shabanzadeh M .Extraction and Determination of Trace Copper (II) Using Octadecyl Silica Membrane Disks Modified 1-(2-Pyridyl Azo) 2-Naphtol(Pan) in Water Samples and Paraffin-Embedded Tissues from Liver Loggerhead Turtles Specimens by FAAS. Jf Chem Health Risks. 2(2),7-18. 2012.
- Choi Y.S.; Choi H.S .High Functional Inorganic Polymers Containing Main Group 13 16 Elements in the Polymer Backbone Chain Bull Korean Chem Soc. 24, 222-228. 2003.
- Unger K .Comparison of an ordered mesoporous aluminosilicate, silica, alumina, titania and zirconia in normal-phase high-performance liquid chromatography Porous Silica. Elsevier, Amsterdam. 1979.
- Boudreau S.P., Cooper W.T. Analysis of thermally and chemically modified silica gels by heterogeneous gas-solid chromatography and infrared spectroscopy. Anal Chem. 61;41-47. 1989.
- Kvitek R.J., Evans J.F., Carr P.W. Denaturation of purple membranes at the air/water interface studied by SEM. Anal Chim Acta. 144, 93-98. 1982.
- Moghimi A. Chinese Journal of Chemistry, 25, 640. 2007
- Mahmoud M.E. Silica gel-immobilized Eriochrome black-T as a potential solid phase extractor for zinc (II) and magnesium (II) from calcium (II).Talanta. 45, 309-314. 1997.
- Mahmoud M.E., Soliman E.M. Study of the selective extraction of iron (III) by silica-immobilized 5-formyl-3-arylazo-salicylic acid derivatives .Talanta. 44, 1063-1069. 1997.
- Mahmoud M.E. In: Proceeding of the 25th FACSS Conference, Austin, TX, USA, 11–15 October. 1998.
- Tong A., Akama Y., Tanaka S.. Selective preconcentration of Au (III), Pt (IV) and Pd (II) on silica gel modified with γ-aminopropyltriethoxysilane. Anal Chim Acta. 230, 179-186. 1990.
- Moghimi A .Separation of Vanadium (V) paraffin-embedded tissues from liver loggerhead turtles specimens by organic solution processable functionalized-nano graphene prior to determination by flame atomic absorption spectrometry. Afr J Pure Appl Chem. 7(2), 79-90.2013.
- Moghimi A .Preconcentration and Determination of Fe(III) Using Octadecyl Silica Membrane Disks and Flame Atomic Absorption Spectrometry. Oriental J Chem. 22(3), 527-535. 2006.
- Wang H., Zhang S., Cheng J.K. Studies on 2-(2-thiazolylazo)-5-diethylaminophenol as a precolumn derivatizing reagent in the separation of platinum group metals by high performance liquid chromatography.Talanta. 48, 1-8. 1999.
- Smith M.B., March J. March’s advanced organic chemistry: reactions,mechanisms, and structure. New York: John Wiley & Sons Inc. 1182–3. 2001.
- Moghimi A., Abedin A.R. Shahriar Ghammamy S., Ghiasi R. Solid phase extraction of Cd (II) using mesoporous organosilicas and determination by FAAS. Afr J Pure Appl Chem. 3(3),051-059. 2009.
- Harvey D., Clifford C.H. Bis (N, N’ – Disalicylalethylenediamine) -µ – quodicobalt(II). Inorg Synth Inorganic Syntheses. 3, 196–201. 1950.
- A.Moghimi. Separation of Trace Amount Zn (II) Using Additional Carbonyl and Carboxyl Groups Functionalized-Nano Graphene Journal of Chemical Health Risks. 2(4): 45-51. 2012.
- Moghimi,A.,Yari,M. Preconcentration of trace Ni (II) using C18 disks nano graphene with amino propyltriethoxysilane (APTES), Merit Research Journal of Environmental Science and Toxicology. 2(5),110-119. 2014.
- Moghimi, A., Akbarieh, S.P. Evaluation of Solid-phase Extraction Sorbent with Octadecane-functionalized Nano Graphene (ODG) for the Preconcentration of Chromium Species in Water, International Journal of Scientific Research in Knowledge. 2(1), 8-21. 2014.
- Moghimi, A. Detection of trace amounts of Pb(II) by schiff base-chitosan-grafted multi-walled carbon nanotubes, Russian J. Physic. Chem. A, 87(7),1203-1209. 2013.
- Su, X.G., Wang, M.J., Zhang, Y.H., Zhang, J.H., Zhang, H.Q., Jin, Q.H. Separation and preconcentration procedures for the determination of lead using spectrometric techniques: A review , Talanta,59,989-995. 2003.
- Soylak, M. ,Karatepe, A.U.,Elci, L., Dogan, M. ,Column preconcentration/separation and atomic absorption spectrometric determinations of some heavy metals in table salt samples using amberlite XAD-1180.Turkish Journal of Chemistry. 27(2),235-242. 2003.
- Szabo, T., Berkesi O., Dekany I. DRIFT study of deuterium-exchanged graphite oxide, Carbon. 43,3186–9. 2005.
- Moghimi, A. Extraction of Ni (II) on micro crystalline naphthalene modified with organic-solution-processable functionalized nano graphene, Russian Journal of Physical Chemistry A. 88(7);1177-1183. 2014.
- Moghimi, A., Abdouss, M. Extraction of Co (II) by Isocyanate Treated Graphite Oxides (iGOs) Adsorbed on Surfactant Coated C18 Before Determination by FAAS ,Int. J. Bio-Inorg. Hybd. Nanomat, 2(1);319-327. 2013.
- Moghimi, A., Sabertehrani, M. , Waquif-Husain S. Preconcentration and determination of chromium species using octadecyl silica membrane disks and flame atomic absorption spectrometry, Chinese Journal of Chemistry. 25(12);1859-1865. 2007.
- Tarigh, G.D. , Shemirani, F. Magneticmulti-wall carbon nano tubenano composite as an adsorbent for preconcentration and determination of lead(II)and manganese (II)in variousmatrices , Talanta. 115,744–750. 2013.
- Moghimi, A., Poursharifi, M.J. Perconcentration of Ni (II) from Sample Water by Modified Nano Fiber, Oriental Journal of Chemistry , Orient. J. Chem. 28(1);353-356. 2012.
- Moghimi, A. Separation and extraction of Co (II) using magnetic chitosan nanoparticles grafted with β-cyclodextrin and determination by FAAS, Russ. J. Phys. Chem. A. 88(12);2157-2164. 2014.
- Moghimi, A., Siahkalrodi, S.Y. Extraction and Determination of Pb (II) by Organic Functionalisation of Graphenes Adsorbed on Surfactant Coated C18 in Environmental Sample, Journal of Chemical Health Risks. 3(3),01-12. 2013.
- Moghimi A. Separation of Vanadium(V) paraffin-embedded tissues from liver loggerhead turtles specimens by organic solution processable functionalized-nano graphene prior to determination by flame atomic absorption spectrometry. Afr J Pure Appl Chem. 7(2);79-90. 2013.
- A. Moghimi, S. Yousefi Siahkalrodi. Extraction and Determination of copper(II) by Organic Functionalisation of Graphenes Adsorbed on Surfactant Coated C18 in Environmental Sample. Journal of Chemical Health Risks. 3(3):01-12. 2013.

