Introduction
While planning the implementation of wastewater treatment; environmental impact studies, treatment objective, levels and efficiency must be clearly addressed [1]. Among toxic substances reaching hazardous levels are heavy metals. Their environmental and health impact on man has raised the objective to efficiently remove these contaminants from wastewater. Indiscriminate release of wastewater containing heavy metals into sewerage and and water bodies by small and medium-scale industries in the major source of a scale heavy metal pollution of water [2, 3].
Some industries, probably due to financial constraints as regards right treatment of their wastewater tend to discharge their effluents in a non-environmental-friendly manner. The pollutants of concern include lead, chromium, gold, iron, silver, copper, nickel etc. These metals may have been derived from mining operations, refining ores, sludge disposal, fly ash from incinerators, processing of radioactive materials, metal plating or the manufacture of electrical equipments, paints, alloys, batteries, pesticides or preservatives [3]. The devastating/toxic effects of heavy metals to the human health and environment, and that of other organisms has been widely reviewed and reported [4, 5, 6, 7, 8]. One of the sustainable ways to avert this is by removing this dangerous species or reducing them to safe amount in discharges and wastewater before they are allowed to enter the water body. The word “sustainable” will remain in the earlier statement only if extensive research into developing alternatives, efficient and sustainable technologies for the removal of these species [9]. Over the years, several methods have being employed for the removal of heavy metals in wastewater. Some of these methods include precipitation, ion exchange, reverse osmosis, phytoremediation e.tc [10, 11].
However, of all the methods listed above, this research work is targeted at the use of chemical precipitation as a technique for treating wastewater. A general guideline on how to remove dissolved toxic metals from wastewater is given and efforts are geared towards providing a sound understanding of the precipitation processes as applied to industrial wastewater treatment. The process ensures a transformation of the dissolved contaminants into insoluble solids, which could be easily removed from the liquid phase by sedimentation or filtration. This process usually involves the adjustment of pH, addition of precipitants and flocculants, if found necessary. The metal precipitation process is dependent, basically on; the concentration of metal ions in the water, the pH, the precipitant, the precipitation time and temperature. Toxic metals are usually present in wastewater in dilute quantities (1-100mg/L) and at neutral or acidic pH values (<7.0) [12, 13].
The research work is aimed at the efficient removal of heavy metal ions form wastewater prior to other treatments; using some cheap and common chemical compounds as precipitant at selected precipitation conditions. This is to provide a cheap and easy alternative for the removal of heavy metal ions in wastewater to be discharged into sewage systems.
Materials and Method
The reagents used which includes; sodium hydroxide, calcium hydroxide, sodium trioxocarbonate (IV), hydrochloric acid, lead chloride, copper chloride, zinc chloride, nickel chloride and iron chloride are products of the British Drug House (BDH).
Preparation of heavy metal solutions from their salts
Stock solutions (1 g/L) of copper (2.683 g of CuCl2.2H20 in 1 litre of distilled water), zinc (2.085 g of ZnCl2 in 1 litre of distilled water), lead (1.343 g of PbCl2 in 1 litre of distilled water), iron (2.907 g of FeCl3 in 1 litre of distilled water) and manganese (2.291 g of MnO2 in 1 litre of distilled water) were prepared. The synthetic wastewaters were then prepared from the stock solutions, while 5 ml each of these stock solutions was introduced into a 1000 ml volumetric flask. The solution was now made up to 1000 ml by adding distilled water to the set mark on the volumetric flask. Aliquots (50 ml) of these were digested using aqua regia, to ensure that the concentrations of the metals of interest in the sample did not diminish. The concentrations of the metals were now measured using the Atomic Absorption Spectrophotometer (Buck Scientific, model: VGP 210).
 |
Precipitation Processes and Final Analyses
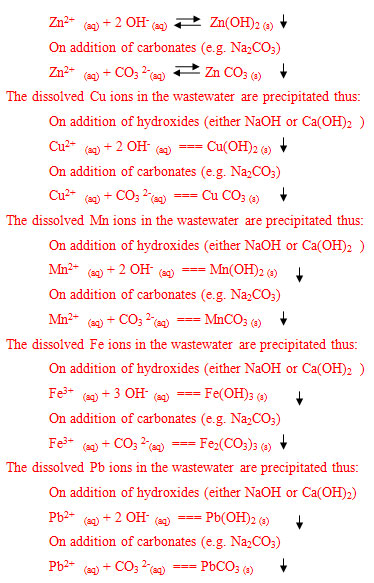
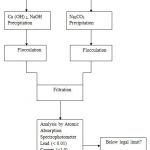
The precipitation was a modification of the method reported by Ayres et al., [14]. The prepared wastewater (100 ml) was poured into a beaker. The pH value was adjusted to approximately 9.0 by slow addition of 12 wt % Ca (OH)2. The solution was heated was slowly heated while stirring, and kept at 50 0C in the water bath. The solution was stirred for about 45 minutes and the precipitate formation was being observed. The stirring was stopped and the precipitate formed was allowed to settle down. The precipitate was removed by filtration using the filter paper. The filtrate (i.e. the treated water) was later digested (to preserve the metal ion concentration) and taken for final analysis. Same was repeated for NaOH, while the pH was adjusted to 10 for Na2CO3.
Result and Discussion
The chemistry of precipitation processes can be summarized as follows;
The dissolved Zn ions in the wastewater are precipitated thus:
On addition of hydroxides (either NaOH or Ca(OH)2)
The precipitate formed is in equilibrium 0077ith ions in the solution. Therefore continuous removal of the precipitate favors efficient remediation of the wastewater. This equilibrium can be described by the solubility product Ksp of the compound formed. The lower the solubility product at the precipitation temperature, the more precipitate is formed, hence a more efficient removal of the heavy metals.
Analysis of the dissolved metal ions in the wastewater
Though similar quantity of the metal salts was used in spiking the water, initial concentrations of metal ions as shown on Table 3, 4 and 5 differs. This is due to the fact that the salts of the heavy metals have varying solubilities in water.
From Tables 3, 4 and 5, its shown that there exist variations in the percentage removal of the metal ions from the water sample. This is due to the fact that each metal has its own optimum precipitation pH [14]. This also accounts for the difference in the concentration of metal ions that were still dissolved in the water, even after the precipitation process.
The Figure 2 show that the adopted pH of precipitation (i.e. 9.0, for NaOH and Ca (OH)2) was most favourable for Cu2+ precipitation than for other metal ions, as this could be proved from the high percentage removal of the metal experienced (i.e. 99.2 % and 100 % respectively) from the solution.
The percentage removal obtained using the Na2CO3 precipitation is averagely low except for Fe3+ with a removal of 97.6 % (Figure 2). This is in agreement with the proposition of Christoph and Anja [13], that carbonates of these metals have high tendencies to re-dissolve after initial precipitation.
Comparing the final concentrations obtained after the precipitation processes with available standards for allowable limits of metal ions in water, it would be seen that the water was adequately treated by the NaOH and Ca(OH)2 precipitation processes, except for Pb2+, which required an extreme level of removal (i.e. to 0.01 mg/L). This actually would be compensated for, when the water is finally diluted, on mixing with a large body of water.
Also, when compared (Figure 2), it was observed that the percentage removal obtained in the Ca(OH)2 precipitation processes is better than that of NaOH except for the removal of lead. This can be attributed to the two OH– that can be dissociated from Ca(OH)2 instead of one from NaOH. The difference in the percentage removal obtained in the Na2CO3 precipitation when compared with the NaOH and Ca(OH)2 precipitation processes is as a result of the different behavior of the hydroxides and carbonates of metal ions. The carbonates of metals are known to have low solubility products [13], which make them easily redissolve into solution even after an initial precipitation while metal hydroxides tend to remain undissolved for as much as the solution is maintained at the optimum pH of precipitation.
Table 1: Results obtained from Calcium hydroxide (Ca (OH)2) precipitation
| S/N | METAL ION | INITIAL CONCENTRATION (mg/L) | FINAL CONCENTRATION (mg/L) | % REMOVAL |
| 1.0 | Pb2+ | 3.20 ±0.08 | 0.90 ±0.02 | 71.9 |
| 2.0 | Cu2+ | 3.76 ±0.10 | ND ≈ 0.00 ±0.00 | 100.0 |
| 3.0 | Mn2+ | 3.09 ±0.07 | 0.27 ±0.01 | 91.3 |
| 4.0 | Zn2+ | 3.07 ±0.06 | 0.54 ±0.03 | 82.4 |
| 5.0 | Fe3+ | 3.72 ±0.08 | 0.25 ±0.02 | 93.3 |
Media temperature = 50 0C, pH=9.0 ND = Not detected
Table 2: Results obtained from Sodium Hydroxide (NaOH) precipitation
| S/N | METAL ION | INITIAL CONCENTRATION (mg/L) | FINAL CONCENTRATION (mg/L) | % REMOVAL |
| 1.0 | Pb2+ | 3.20 ±0.05 | 0.20 ±0.01 | 93.8 |
| 2.0 | Cu2+ | 3.76 ±0.01 | 0.03 ±0.01 | 99.2 |
| 3.0 | Mn2+ | 3.09 ±0.04 | 0.66 ±0.01 | 78.6 |
| 4.0 | Zn2+ | 3.07 ±0.04 | 0.76 ±0.03 | 75.2 |
| 5.0 | Fe3+ | 3.72 ±0.04 | 0.27 ±0.01 | 92.7 |
Media temperature = 50 0C, pH= 9.0
Table 5: Results obtained from Sodium trioxocarbonate (IV) (Na2CO3) precipitation
| S/N | METAL ION | INITIAL CONCENTRATION (mg/L) | FINAL CONCENTRATION (mg/L) | % REMOVAL |
| 1.0 | Pb2+ | 3.20 ±0.04 | 0.80 ±0.00 | 75.0 |
| 2.0 | Cu2+ | 3.76 ±0.10 | 0.91 ±0.02 | 75.8 |
| 3.0 | Mn2+ | 3.09 ±0.04 | 0.78 ±0.01 | 74.8 |
| 4.0 | Zn2+ | 3.07 ±0.08 | 0.64 ±0.01 | 79.2 |
| 5.0 | Fe3+ | 3.72 ±0.10 | 0.09 ±0.00 | 97.6 |
Temperature of precipitation= 50 0C, pH=10
Mixing time in each precipitation= 45 minutes.
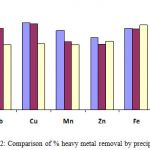 |
Figure 2: Comparison of % heavy metal removal by precipitant |
Conclusion
NaOH, Ca(OH)2 and Na2CO3 are capable of precipitating heavy metals including Pb2+, Cu2+, Mn2+, Zn2+ and Fe3+ at the selected precipitations conditions. The variation in the percentage removal of the heavy metals has been attributed to the nature of the heavy metal ions, nature of the cations from the precipitants, amounts and nature of anions for the precipitants and the solubility of the hydroxides and carbonates at the selected precipitation conditions. At the selected conditions, Cu2+ was completely precipitated out of it solution by Ca(OH)2. Other metal ions were also observed to have been reduced to the least threatening amounts in the solutions after their precipitation. This presents new set of conditions at which the selected precipitants can be utilized in the treatment of some heavy metal polluted water.
References
- Sperling, M. IWA publishing. 163, 2007.
- Pino, G. H., de Mesquita, L. S., Torem, M. L., & Pinto, G. A. S. Separation Science and Technology. 41(14), 3141-3153, 2006 .
- Osemeahon S.A., Nkafamiya I.I., Anioke D., Akinterinwa A., Adebayo I.T. Journal of water pollution and purification research. 3(3) 23-28, 2016.
- Ansari, M. I., Malik, A. Bioresource technology. 98(16), 3149-3153, 2007.
- Bhattacharya, A. K., Mandal, S. N., & Das, S. K. Chemical Engineering Journal. 23(1), 43-51, 2006.
- Parvathi, K., Kumar, R. N., & Nagendran, R. World Journal of Microbiology and Biotechnology. 23(5), 671-676, 2007.
- Deng, L., Su, Y., Su, H., Wang, X., & Zhu, X. Adsorption.12(4), 267-277, 2006.
- Borba, C. E., Guirardello, R., Silva, E. A., Veit, M. T., & Tavares, C. R. G.Biochemical Engineering Journal. 30(2), 184-191, 2006.
- Akinterinwa A., Fasina E.O., Joseph J. and Aminu A. Asian Journal of Chemical Sciences. 3(2): 1-10, 2017.
- Blanco, A., Sanz, B., Llama, M. J., & Serra, J. L.Journal of Biotechnology. 69(2), 227-240, 1999.
- Akinterinwa A., Nkafamiya I.I., Mustapha A., Japari J. I. Journal of Water Pollution & Purification Research, 3(1) 6-13, 2016.
- Kim, B. R., Gaines, W. A., Szafranski, M. J., Bernath, E. F., & Miles, A. M.Journal of environmental engineering.128(7), 612-623, 2002.
- Christoph Pasel and Anja Elsner. University of Duisburg. Duisburg. 2-5, 2006.
- Ayres, D. M., Davis, A. P., Gietka, P. M. Engineering Research Centre Report. 90, 1994.