Introduction
Mushrooms are an assemblage of fleshy macroscopic fungi. They possess a distinctive fruiting body that could by hypogenous or epigeous, large enough to be seen by naked eyes & to be picked by hands. Edible mushrooms are ideal low calorie foods for diabetic patients since they contain very low amounts of fats cholesterol low levels of carbohydrates, high content of protein, vitamins & minerals. Mushrooms are known to contain compounds which help in proper functioning of the liver, Pancreas & other endocrinal gland thereby promoting formation of insulin & related hormones which ensure healthy metabolic functioning. Polysaccharides, such as beta glucans contained in mushrooms have the ability to restore the function of pancreatic tissues by causing increased insulin output by beta-cells which leads to lowering of blood glucose levels. It has also been shown to improve the sensitivity of peripheral tissues to insulin. Consumption of mushrooms markedly decreases the lipid levels including total cholesterol, total triglyceride, & low- density lipoproteins & increase the level of high- density lipoproteins [1]. Medicinal mushrooms and their constitutive active compounds have been described to have reducing many diseases including cancer, hypertension, metabolic syndrome and cardiovascular diseases. Many studies have focused on their immunomodulatory and anti-tumor effects because mushrooms may contain a diverse array of biologically active metabolites (β-D-glucans, immunomodulatory proteins, secondary metabolites) with well-known immune enhancing capabilities. Some chemical and biochemical hypoglycemic agents (anti-diabetes agents), such as insulin, Metformin, tolbutamide, gliclazide, phenformin, troglitazone and rosigitazone, exenatide are the mainstay in the treatment of diabetes and are effective in controlling hyperglycemia. However, these anti-diabetes agents may have harmful side-effects, fail to significantly alter the course of diabetic complications and there is insufficient knowledge on the pharmacological management of the disease .Therefore, natural antidiabetic drugs from medicinal plants have attracted a great deal of attention. Admittedly, diabetes is a metabolic disorder which should be controlled or prevented with appropriate lifestyle adaptations including exercise, appropriate food and health relevant environments. Indeed healthy foods rich in various medicinal properties provide a means to good health. Edible and medicinal mushrooms are functional foods and thus a good solution to controlling diabetes and a potent source of biologically active compounds with anti-diabetic effects. Many mushroom species appear to be effective for both the control of blood glucose levels and the modification of the course of diabetic complications. Agaricus bisporus is a popular edible mushroom worldwide. The mushroom has potential anti-inflammatory, hypoglycemic and hypocholesterolemic effects due to presence of high amounts of acidic polysaccharides, dietary fibre, and antioxidants, such as vitamins C, B12, and D; folate, ergothioneine; and polyphenol. The increased intake of white button mushrooms may promote innate immunity against tumors and viruses High concentrations of blood cholesterol levels, hypercholesterolemia, can lead to a progression of hyperglycemia/ type 2 diabetes in humans and animals. Cholesterol directly effects β-cell metabolism and opens a novel set of mechanisms that may contribute to β-cell dysfunction and the onset of diabetes. Epidemiological studies suggest that higher levels of dietary fibre intake play a significant protective role with respect to diabetes, in lowering the dietary glycemic load and shows potent hypocholesterolemic effects. Diabetic rats fed A. bisporus fruiting bodies exhibited significant anti-glycemic and anti-hypercholesterolemic effects. Moreover, the mushrooms had a positive influence on lipid metabolism and liver function. Although soluble dietary fibre is the most likely candidate in lowering blood glucose levels and cholesterol levels, other constituents, such as anti-oxidants (polyphenol, vitamin C, and ergothioneine), proteins, and polysaccharides may also play an important role.[2]
Agaricus -Taxonomic position
Division: Mycota
Sub-division: Eumycotina
Class: Basidiomycete
Subclass: Homobasidiomycetidae
Series: Hymenomycetes
Order: Agaricales
Family: Agaricaceae.
Genus: Agaricus
Species: Bisporus
 |
Most widely cultivated species for food purposes is Agaricus Bisporus in India [3]
Cultivation And Cllection
Favorable season: Oct. to March (for plains of India)
Required temp.and humidity:14-220C and 80-85%
Cultivation process involves four major steps
- Preparation of compost
- Spawning of compost
- Casing (Covering the spawned compost)
- Cropping and crop management
Preparation of compost
Unlike other traditional crops soil is not the appropriate substrate for mushroom cultivation. Rather, the substrate for mushroom called compost, is prepared from agro wastes like straw, stem, shoot, apices etc. with organic manure. Mushroom substrate may be simply defined as a lingo-cellulosic material that supports the growth, development and fruiting of mushroom mycelium. This compost is pasteurized by various micro-organisms and at appropriate temperature range. Essential supplement are also added/ supplemented to the compost. The whole process is termed as composting. Generally composting refers to the piling of substrates for a certain period of time and the changes due to the activities of various micro-organisms, which result in a composted substrate that is chemically and physically different from the starting material. The compost provides nutrients, minerals, vitamins and ions required for proper growth of mushroom. This compost supports the growth of only the mycelium of button mushroom and prevents that of other competitive moulds.
Methodology for compost preparation
Compost is an artificially prepared growth medium from which mushroom is able to derive important nutrients required for growth and fructification. Cemented floors are required for making good quality compost. There are two main methods for compost preparation:
Long method of composting
This is an outdoor process and takes around 28 days in its completion with a total of seven turnings.
Before making compost, wheat straw is spread on cemented floor and is turned many times with water being spread at regular intervals.
Day 0
At the stage, there should be around 75% humidity content in the wheat straw, to which wheat bran, calcium ammonium nitrate, urea, murate of potash, and super phosphate are mixed thoroughly and evenly. The material is then piled 1.5m thick x1.25m high with the help of wooden rectangular block. The blocks are removed. Once the entire material has been stacked up or piled up. Water is sprayed twice or thrice to keep the substrate moist. Temperature should be in the range of 70-750C.
1st turning Day 6
On the sixth day first turning is given to the stack. The purpose of turning is that every portion of the pile should get equal amount of aeration and water. If the turnings are not given, then anaerobic condition may prevail which may lead to the formation of non-selective compost. In the stack, the central zone is fermenting at its peak and has maximum temperature rest of the portion is either not at all fermented or ferments improperly. The correct method of turning is as: Removing about 15cm of compost from the top and spread it on one side of the floor, the rest part of compost on the other side of the floor. Now turning is done by shaking the outer (top most) part and the inner part of the compost, first separately and then missing them altogether thoroughly with the help of wooden buckets.
2nd turning (Day 10)
On the tenth day, again the top most part and the inner part of the compost is separated, water is sprayed on the top part. Again the two parts are piled up together in such a way that now the top part is inside and the inner part is on the top of the stack.
3rd turning (day 13)
it is also done in the same way as described earlier. Gypsum and furadan are mixed at this stage.
4th turning (day 16)
The same process of turning is followed.
5th turning (day 19)
The compost is turned in the same manner and B.H.C. is added.
6th turning (day 22)
The same process of turning is followed.
7th turning (day 25)
If no ammonia persists in the compost, spawning is done
Short method of composting
Compost prepared by short method composting is superior in production quality and the chances of infection and disease is quite low.
This method is accomplished in two phases
Phase I- Outdoor composting
Wheat straw mixed with chicken manure is sprayed with water and a 45cm high pile is made on the fourth day and first turning is made. On 7th day, wheat bran, gypsum and urea is mixed thoroughly and piled up to 1.25-1.50 m height with a width ranging from 1.25 -1.5 m. The internal temperature of the compost should be maintained at 70-750C within 24hr. Second turning is done on this day where as third turning is done on 8th day with subsequent mixing of gypsum. On the 10th day, the compost is transferred to the pasteurization tunnel. Compost is filled in the pasteurization tunnel to a height of 7’. Filling height depends upon the size of the tunnel.
Phase II- Indoor composting
This is the pasteurization procedure which is done in a closed environment. Pasteurization has got many purposes.
- If the temperature during composting has been low and the compost is heterogeneous, many parasites (nematodes, pathogens, flies and mites etc.) will survive in the compost mass, therefore, pasteurization is the best means with which these parasites can be destroyed.
- To end fermentation and to convert compost into a chemical and biological state favourable to the development of the mycelium and unfavourable to moulds.
- Conversion of ammonia into microbial protein.
Compost is filled in the pasteurization tunnel and as soon as the compost in the tunnel is completely filled the doors and fresh air damper are properly closed and blower is put on for recirculation of air @ 150-250 cubic metre/ 1000 kg compost/ hour. The phase II process is completed in three stages:
Pre-peak heat stage
After about 12-15 hours of compost filling, the temperature of compost starts rising and once 48-500C is obtained, it should be maintained for 36-40 hours with ventilation system. Normally such temperature is achieved by self generation of heat by the compost mass without steam injection.
Peak heat stage
Raise the temperature of compost to 57-580C by self generation of heat from microbial activity if it is not obtained. Injecting the live steam in the bulk chamber and maintain for 8 hours in order to ensure effective pasteurization. Fresh air introduced by opening of the fresh air damper to 1/6 or 1/4 of its capacity and air outlet too is opened to the same extent.
Post- peak heat stage
lower down the temperature gradually to 48-520C and maintain till no traces of ammonia are detected in compost. This may take 3-4 days in a balanced formulation. When the compost is free from ammonia, full fresh air is introduced by opening the damper to its maximum capacity and cool down the compost to around 250C which is considered as the favourable temperature for spawning.
Spawning
The process of mixing of the spawn in the compost is known as spawning. Spawn is thoroughly mixed in the compost at the rate of 600-750 gm per 100 kg of compost (0.6 – 0.75%). The spawned compost is filled in tray or polypropylene bags covered with formalin treated news papers. In case of bags, they should be folded at the top and covered up. After spawning, temperature and humidity of crop room should be maintained at 18-220C and 85-90%, respectively. Water should be sprayed over the covered news papers, walls and floors of the crop room. After 12-14 days of spawning white mycelial growth is seen running the entire length of the tray/bag. This is then covered with casing soil on the surface.
Casing soil
The significance of casing soil is to maintain the moisture content and exchange of gases within the surface of the compost which helps in the proper growth of the mycelium. The pH of the casing soil should be 7.5-7.8 and must be free from any infection or disease.
Pasteurization of casing soil
The casing soil is piled on cemented floor and is treated with 4% formalin solution. Thorough turning of the soil is done and it is covered with polythene sheet for the next 3-4 days. Pasteurization of casing soil at 650C for 6-8 hours is found to be much more effective.
Using the casing soil
3-4cm thick layer of casing soil is being spread uniformly on the compost when the surface has been covered by white mycelium of the fungus. Formalin solution (0.5%) is then being sprayed. Temperature and humidity of the crop room should be maintained at 14-180C and 80-85%, respectively. Proper ventilation should be arranged with water being sprayed once or twice a day.
Harvesting of crop
Pin head initiation takes place after 10-12 days of casing and the fruiting bodies of the mushroom can be harvested for around 50-60 days. The crops should be harvested before the gills open as this may decrease its quality and market value.
Productivity
From 100 kg compost prepared by long method of composting 14-18 kg of mushroom can be obtained. Similarly, 18-20 kg mushroom can be obtained from pasteurized compost (Short Method Compost)[4]
Phytochemical compounds
Literature indicates that mushrooms have phytochemicals compound such as Alkaloid, Carbohydrates, steroids, glycosides, flavonoids, protein, amino acids, phenols, Saponins, triterpenoids presented[5],[6]
Pantothenic acid[7], Riboflavin[7], Niacin[7], Vitamin C[7], Chitin [8],Beta glucan [8], Vitamin D[9]
 |
Pharmacological Activities
Anti diabetic
Mushrooms are known to contain compounds which help in proper functioning of the liver pancreas and other endocrinal glands, thereby promoting formation of insulin and related hormones which ensure healthy metabolic functioning. Polysaccharides, such as beta glucans contained in mushrooms have the ability to restore the function of pancreatic tissues by causing increased insulin output by β – cells, which leads to lowering of blood glucose levels. It has also been shown to improve the sensitivity of peripheral tissues to insulin. Consumption of mushrooms markedly decreases the lipid levels including total cholesterol, total triglyceride, and low-density lipoproteins; and increases the level of high-density lipoproteins.[10]
Antioxidant
Mushrooms also contain antioxidant compounds such as polyphenols and flavones that are involved in antioxidant defence for humans, Such compounds have the ability to block reactive oxygen species involved in lipid peroxidation, oxidative stress that leads to DNA, cell membrane proteins and cellular organelles damage. Oxidative stress arises from an imbalance between the production of reactive oxygen species and antioxidant systems in the human body, when the ability to inactivate these compounds is low.[11]
Antimicrobial and antiviral activity
The ethanolic extract of fruiting bodies of A. bisporus contains various components with antimicrobial activity. The freeze-dried extract of this species displays activity towards Escherichia coli CBAB 2 (Minimum Inhibitory Concentration – MIC – 5 mg/mL), and also against Staphylococcus aureus ATCC 6588 (Gram negative). On the other hand, Pseudomonas aeruginosa ATCC 15442 has proved to be the most resistant strain, with a MIC value of 15 mg/mL. The antimicrobial action of numerous mushroom species (including A. bisporus) is due to high contents of chitosan and chitin. Chitin and its deacetylated derivative – chitosan, are polysaccharides whose molecular weight is relatively high (similar to the ones found in crustaceans), which could suggest that their antibacterial properties are reduced. The antimicrobial effect of chitin and its derivatives increases with a decrease in the molecular weight. The activity is based on decreasing bacterial adhesion to the culture medium into Agaricus campestris, a species closely related to A. bisporus, has shown the presence of agarodoxin – a benzoquinone derivate. This substance is an antibiotic and shows activity against Staphylococcus aureus (golden staph).
Anti-carcinogenic
A.bisporus, including hydroxybenzoic acid and protocatechuic acid, are also characteristic for this species. These substances, apart from their typical anti-carcinogenic activity. The application of A. bisporus to anticancer therapy should, therefore, be feasible and inexpensive.[12]
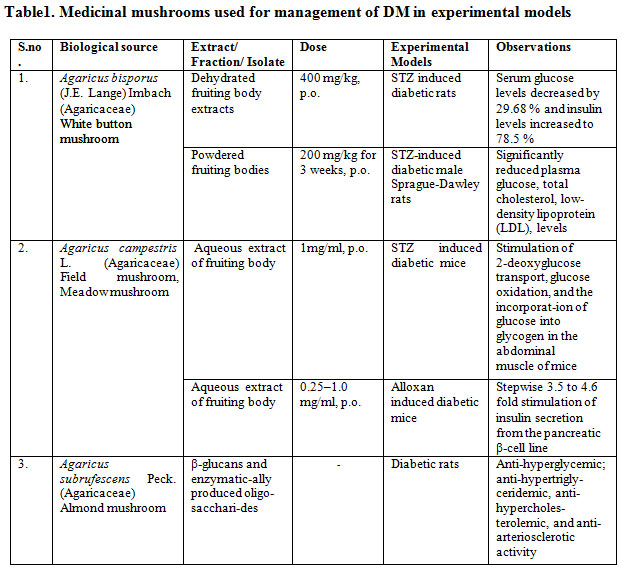
Summary of studies demonstrating the anti-diabetic effects of several medicinal mushroom in experimental models as well as in clinical studies are shown in table 1 and 2 respectively [10]
Table 1: Medicinal mushrooms used for management of DM in experimental models
| Hot water extract
of the submerged- culture broth (ethyl acetate fraction) |
200 and 400
mg/ kg, p.o. |
Diabetic male
Sprague-Dawley rats |
Reduced blood glucose
level and elevated plasma insulin and glucose transport-4 proteins |
||
| Powdered
fruiting bodies |
1g/kg for 2
months, p.o. |
STZ-induced
diabetic rats |
Significant suppression of
increased fasting plasma glucose; increased Serum insulin levels |
||
| 4. | Agrocybe
cylindracea (DC.) Maire (Strophariaceae) Chestnut Mushroom, Poplar mushroom |
A glucan (AG-
HN1) and a heteroglycan (AG-HN2) isolated from hot-water extract of the fruiting bodies |
I.p. | Normal and
STZ-induced diabetic mice |
AG-HN1 showed a
remarkable hypoglycemic activity in both normal and STZ-induced diabetic mice, higher than that of AG-HN2 |
| 5. | Astraeus
hygrometricus (Pers.) Morgan (Diplocystaceae) False earthstar |
Ethanolic extract
of fruiting bodies |
250,500, 1000
mg/kg, p.o. |
Alloxan induced
diabetic mice |
Reduced levels of blood
glucose; better tolerance to glucose |
| 6. | Auricularia
auricula-judae (Bull.) J. Schrot. (Auriculariaceae) Jew’s Ear, Jelly Ear mushroom |
Water-soluble
poly-saccharide from fruiting bodies |
30 g/kg; in
diet |
Genetically
diabetic KK-Ay mice |
Significant effect in
lowering plasma glucose, insulin, urinary glucose, and food intake; increased tolerance to intraperitoneal glucose loading and the hepatic glycogen content |
| Hot water extract
from fruiting bodies |
Diet
containing 5% extract |
Genetically
diabetic (type 2) KK-Ay |
Reduced postprandial
hyperglycemia |
||
| Dried mycelia
powder |
0.5 and
1.0g/kg, p.o. |
Genetically
diabetic mice |
Significant reduction of
plasma glucose, total cholesterol and triglyceride levels |
||
| 7. | Coprinus comatus
(O.F. Mull.) Pers.(Coprinaceae) Shaggy ink cap |
Powdered dried
fruiting bodies |
Diet with
33.3% w/w powder |
Normal mice | Reduced Plasma glucose;
improved intraperitoneal glucose tolerance |
| Fermented
mushroom rich in vanadium |
i.g. route | Normal,
Alloxan and adrenalin induced hyperglyc-emic mice |
Decreased blood glucose
levels; improved sugar tolerance of normal mice |
||
| 4,5- Dihydroxy-
2- methoxybenzalde hyde (comatin) isolated from fermentation broth |
80 mg/kg,
p.o. |
Normal and
alloxan induced diabetic rats |
Inhibition of the non-
enzymatic glycosylation (NEG) reaction; decreased concentrations of fructosamine,triglycerides and total cholesterol. Maintained levels of blood glucose and improved glucose tolerance |
| 8. | Cordyceps
militaris (L.) Link (Clavicipitaceae) Caterpillar Killer |
Exo-polymers
produced from submerged mycelia cultures |
50mg/kg for 7
days, p.o. |
STZ- induced
diabetic rats |
Significantly decreased
levels of plasma glucose, total cholesterol, triglyceride and plasma glutamate-pyruvate transaminase (GPT) |
| Aqueous fruiting
body extract |
0.5 g/kg in
diet |
Type 2
diabetic rats |
Amelioration of insulin
resistance and improved insulin secretion |
||
| Aqueous fruiting
body extract |
10 g/kg in
diet |
Rats (90% of
pancreas removed) |
Significant
reduction of fasting serum glucose levels, increased body glucose disposal rates and glucose utilization in skeletal muscles |
||
| 9. | Cordyceps sinensis
(Berk.) Sacc. (Clavicipitaceae) Caterpillar fungus |
Polysaccharide
fraction CSP-1, isolated from cultured mycelia |
200 and
400mg/kg/ day for 7 days, p.o. |
Normal; alloxan
and STZ- induced diabetic rats |
Significant drop in blood
glucose levels and increased serum insulin levels, stimulation of pancreatic release of insulin and/or reduced insulin metabolism |
| 10. | Cordyceps.
takaomontana [anamorph: Paecilomyces tenuipes (Peck) Samson] (Clavicipitaceae) |
Aqueous extract
of fruiting bodies |
0.5 g/kg, in
diet for 8 weeks |
90% pancrea-
tectomized male Sprague Dawley rats |
Improvement of insulin
Resistance and insulin secretion |
| Fruiting body
extract containing 4- β- acetoxyscirpe- ndiol (ASD) |
– | – | Decreased blood sugar in
the circulatory system as specific inhibitors of Na+/ glucose transporter-1 (SGLT-1) |
||
| 11 | Fomitopsis
pinicola (Sw.) P. Karst. (Fomitopsidaceae) Red Banded Polypore |
Water extract
(WE) and an alkali extract (AE) from the fruit body |
Dietary
supplementati on |
STZ- induced
diabetic rats. |
AE showed the highest
antidiabetic effect. These results indicate that constituents of F. pinicola may regulate hyperglycemia via either increased insulin secretion during recovery or the prevention of STZ- induced pancreatic damage. |
| 12. | Ganoderma
applanatum (Pers.) Pat. (Ganodermataceae) Artist’s Bracket |
Ganoderma
applanatum exo- polymer (GAE), produced by submerged mycelial cultures |
100 mg/kg,
p.o. for 3 weeks |
STZ-induced
diabetic rats |
Reduced plasma glucose;
plasma total cholesterol and triglyceride levels |
| 13. | Ganoderma
lucidum (Curtis) P.Karst |
Aqueous extract
of fruiting bodies |
500 and 1000
mg/kg, p.o. |
Alloxan induced
and normal Wistar rats |
Significant hypoglycemic
and antihyper- glycemic effects |
| (Ganodermataceae)
Reishi or Lingzhi mushroom |
Aqueous extract
of fruiting bodies (Ethylacetate and n-Butanol fractions) |
50 mg/kg i.p.
daily for two weeks |
Alloxan-induced
wistar rats |
Significant reduction of
fasting blood glucose |
|
| Aqueous extract
of fruiting bodies |
100 and 200
mg/kg, by gavage once daily for four weeks |
Normal and
STZ-induced hyper- glycemic rats. |
Decreased
serum glucose levels; increased serum insulin levels; improved serum lipid profile in both normal and diabetic animals |
||
| Ganoderma
lucidum polysacchari-des (Gl-PS) |
50 mg/kg and
150 mg/kg, p.o. |
STZ induced
diabetic mice |
Significant increase in
body weights and serum insulin levels; decreased fasting blood glucose levels |
||
| Proteoglycan
extract, FYGL (Fudan- Yueyang-G. lucidum), from the fruiting bodies |
40 and 120
mg/kg, p.o. |
STZ induced
type 2 diabetic rats |
Decrease in fasting plasma
glucose and increase in insulin concentration; decreased levels of free fatty acid, triglyceride, total cholesterol and low density lipoprotein cholesterol as well as increased level of high density lipoprotein cholesterol |
||
| 14. | Grifola frondosa
(Dicks.) Gray (Fomitopsidaceae) Hen of the woods, Maitake |
Powdered
fruiting body |
1g/day, p.o. | Genetically
diabetic mouse (KK-Ay) |
Reduced levels of blood
glucose, insulin and triglycerides |
| Ether-ethanol
soluble (ES) and hot water-soluble (WS) fractions from fruiting body |
ES-fraction or
WS-50% ethanol float (X) fraction, p.o. |
Genetically
diabetic mouse (KK-Ay) |
Blood glucose lowering
activity not only in the ES- fraction consisting of lipid but also in the X-fraction of peptidoglycan |
||
| Powdered
fruiting bodies |
20% maitake
solid feed |
Type 2 diabetic
Female KK-Ay mice |
Inhibition of increase in
blood glucose levels |
||
| MT-α-glucan,
from the fruiting bodies |
150-450
mg/kg |
Type 2 diabetic
KK-Ay mice. |
Antidiabetic activity,
related to its effect on insulin receptors (i.e., increasing insulin sensitivity and ameliorating insulin resistance of peripheral target tissues |
||
| Fermented G.
frondosa rich in vanadium (GFRV) |
i.g. route | Alloxan- and
adrenalin- induced hyperglycemic mice |
Significant decrease in
blood glucose levels |
| 15. | Hericium
erinaceus (Bull.) Pers. (Ericaceae) Lion’s Mane Mushroom, Hedgehog Mushroom |
Methanol extract
of fruiting bodies |
100 mg/kg, in
diet |
STZ-induced
diabetic rats |
Decreased blood sugar
levels and lipid levels |
| 16. | Inonotus obliquus
(Ach. ex Pers.) Pilat (Hymenochaetace- ae) Chaga mushroom |
Protein-
containing polysacchari-des, extracted from sclerotia and mycelia |
– | – | Hypo-
glycemic effect |
| Fruiting body
extract |
Chaga 1 (dose
of 0.09 mg/kg), Chaga 5 (5 times of Chaga 1), and Chaga 10 (10 times of Chaga 1) for 6 weeks, p.o. |
Genetically
obese mice |
Fasting blood glucose
level was significantly lower in the Chaga 5 group; glucose-6- phosphatase activity in liver was significantly the lowest in Chaga 10 group |
||
| Dried matter of
culture broth |
500 and 1000
mg/kg, in diet |
Alloxan induced
diabetic mice |
Significant antihyper-
glycemic; antilipid- peroxidative and antioxidant effects |
||
| Ethyl acetate
fraction |
– | Alloxan-induced
diabetic mice |
Significant antihyper-
glycaemic and antilipidperoxidative effects |
||
| 17. | Laetiporus
sulphureus var. miniatus (Jungh.) Imazeki (Fomitopsidaceae) Sulphur polypore |
Crude
extracellular polysaccharides (EPS), produced from submerged mycelial culture |
200 mg/kg for
14 days, p.o. |
STZ-induced
diabetic rats |
Decreased plasma glucose
levels, increased insulin antigenesity via proliferation or regeneration of diabetic islet β-cells |
| 18. | Lentinula edodes
(Berk.) Pegler (Marasmiaceae) Shiitake |
Exopolymers
produced from submerged mycelia cultures |
50 mg/kg for
7 days, p.o. |
STZ-induced
diabetic rats |
Significant reduction in
plasma glucose, total cholesterol and triglyceride levels |
| Exopolymer
produced from submerged mycelia cultures |
200 mg/kg,
p.o. |
STZ-induced
diabetic rats |
Reduced plasma glucose,
total cholesterol and triglyceride levels; increased plasma insulin levels |
||
| 19. | Lentinus strigosus
Fr. (Polyporaceae) Ruddy panus |
Exopolysacch-
arides (EPS) from submerged mycelial culture |
150 mg/kg for
7 days, p.o. |
STZ-induced
diabetic rats |
Decreased plasma glucose
level; induces regeneration of pancreatic islets and remediates destruction of micro-vascular pancreatic islets |
| 20. | Phellinus badius
(Cooke) G. Cunn (Hymenochaetace- ae) |
Aqueous extract
of fruit body and mycelial biomass |
Aqueous
extracts of basidio-carp, and mycelial biomass at the doses of 800 mg/kg and 1000 mg/kg respecti-vely |
Alloxan-induced
diabetic rats. |
Significant reduction in
blood glucose, plasma triglyceride and cholesterol levels; marked reduction in the level of aspartate amino- transferase (AST) and alanine amino-transferase (ALT). |
| 21. | Phellinus baumii
Pilat (Hymenochaetace- ae) |
Crude
exopolysaccharid es from submerged mycelial cultures |
200 mg/kg,
p.o. |
STZ-induced
diabetic rats |
Hypoglycemic effect with
substantially reduced plasma glucose levels |
| Exopolysacch-
arides (EPS) produced by submerged mycelial culture |
200 mg/kg for
52 days, p.o. |
ob/ob mice | Reduced plasma glucose
levels, increased glucose disposal, reduced blood triglyceride levels |
||
| 22. | Phellinus linteus
(Berk. & M.A. Curtis) Teng, Zhong Guo De Zhen Jun (Hymenochaetacea e)Meshimakobu, Song-Gen, Sang- Hwang |
Exo-polymers
from submerged mycelia cultures |
50 mg/kg for
7 days, p.o. |
STZ-induced
diabetic rats |
Reduced plasma glucose,
total cholesterol and plasma glutamate-pyruvate transaminase (GPT) levels |
| Extracellular
polysaccharides extracted from submerged mycelia cultures |
100 mg/kg,
p.o. |
STZ-induced
male Sprague– Dawley rats |
Hypoglycemic effects with
decreased plasma glucose, total cholesterol and triacyl- glycerol concentrat-ion |
||
| Polysaccharide
(PLP) isolated from Phellinus linteus |
– | Non-obese
diabetic (NOD) mice |
Mean blood glucose levels
were 110mg/dl in PLP- treated mice as compared to 499mg/dl in control NOD mice |
||
| 23. | Phellinus merrillii
(Murrill) Ryvarden (Hymenochaetace- ae) |
EtOAc-soluble
fractions of ethanol extract of fruiting bodies |
– | Male Sprague-
Dawley rats |
Strong α-glucosidase and
aldose reductase inhibitory activities |
| 24. | Phellinus ribis
(Schumach.) Quel (Hymenochaetace- ae) |
Polychlorinat-ed
compounds from methanolic extract of the fruiting body |
– | – | Therapeutic effects
through the enhanced PPAR-γ agonistic activity |
| 25. | Phellinus rimosus
(Berk.) Pilat (Hymenochaetace- ae) Cracked cap polypore |
Fruiting body
extract |
50 and 250
mg/kg for 10 days, p.o. |
Alloxan-induced
diabetic rats |
Significant dose-
dependent hypo-glycemic activity |
| 26. | Pleurotus abalonus
Y.H. Han, K.M. Chen & S. Cheng (Pleurotaceae) Abalone mushroom |
Polysaccharide-
peptide complex LB-1b from fruiting bodies |
– | Drug-induced
diabetic mice |
High antioxidant activity
with a significant hypoglycemic effect |
| 27. | Pleurotus | Water-soluble | 0.4 g/kg, in | STZ- induced | Reduced fasting blood |
| citrinopileatus
Singer (Pleurotaceae) Golden oyster mushroom |
polysaccha-rides
(WSPS), extracted from submerged fermented medium |
diet | diabetic rats | glucose levels | |
| 28. | Pleurotus eryngii
(DC.) Quél. (Pleurotaceae) King trumpet mushroom, French horn mushroom, King oyster mushroom, King brown mushroom, Boletus of the steppes, Trumpet royale |
Freeze-dried,
powdered fruiting body |
Diet
containing 5% freeze dried mushroom |
Male db/db
mice |
Reduced total cholesterol,
triglyceride levels, and increased high density lipoprotein cholesterol levels with improved insulin sensitivity |
| 29. | Pleurotus ostreatus
(Jacq.) P. Kumm. (Pleurotaceae) Oyster mushroom |
Powdered
fruiting bodies |
Diet
containing 4 % mushroom |
Type 2 diabetic
rats |
Significantly lower basal
and postprandial glycaemia. |
| Ethanol extract
of fruiting bodies |
250, 500 and
1000 mg/kg |
Alloxan induced
diabetic rats |
Dose dependent decrease
in blood glucose and cholesterol effects |
||
| Ethanol extract
of fruiting bodies |
100 and 200
mg/kg for 30 days, p.o. |
STZ – induced
diabetic rats |
Significant decrease of
blood glucose levels, genetic alterations and sperm abnormalities |
||
| Suspension of
freeze-dried and powdered fruiting body |
250, 500, 750,
1000, and 1250 mg/kg, p.o. |
Normal and
alloxan-induced diabetic Wistar rats |
Significantly reduced
levels of serum glucose. Hypo-glycemic effect comparable with metformin and glibenclamide |
||
| Ethanol extract
of fruiting bodies |
380, 760 and
1140 mg/kg, i.p. |
Alloxan-induced
diabetic rats |
Significant reduction in
blood glucose levels |
||
| Ethanol extract
of fruiting bodies |
– | Normal and
alloxan-induced diabetic mice. |
Significant decrease in
serum glucose level; reduced serum cholesterol, triglyceride and LDL- cholesterol levels |
||
| 30. | Pleurotus
pulmonarius (Fr.) Quel (Pleurotaceae) Indian Oyster, Italian Oyster, Phoenix Mushroom, Lung Oyster |
Aqueous extract
of fruiting bodies |
250, 500, and
1000 mg/kg, p.o. |
Normal and
Alloxan-induced diabetic mice |
Antihyper-glycemic effect
(increased glucose tolerance in both normal and diabetic mice) |
| 31. | Sparassis crispa
(Wulfen) Fr. (Sparassidaceae) Cauliflower fungus |
β-glucan
component |
– | – | An effective promoter of
wound healing in patients with diabetes. Increase in the migration of macrophages and fibroblasts, and directly increased synthesis of type I collagen |
| Freeze dried
fruiting body samples |
Dietary
supplementati on |
Diabetic KK-Ay
mice |
Increased plasma levels of
adiponectine; decreased blood glucose levels, serum triglycerides and total cholesterol levels |
||
| 32. | Stropharia
rugosoannulata Farl. ex Murrill. (Strophariaceae) Wine cap, Burgundy mushroom King stropharia |
Extracellular
polysaccharide (EPS) |
– | STZ- induced
diabetic rats |
Decrease in plasma
concentrations of glucose,total cholesterol, and triacylglycerol; decreased aspartate amino- transferase activity |
| 33. | Trametes gibbosa
(Pers.) Fr. (Polyporaceae) Lumpy bracket |
Extracellular
polysaccharide (EPS) |
– | STZ- induced
diabetic mice |
Decreased plasma glucose,
total cholesterol and triacylglycerol concentrations |
| 34. | Tremella aurantia
Schwein. (Tremellaceae) Golden ear |
Acidic
polysaccharide (TAP) solution and TAP-H (degradation products of TAP) solution |
TAP
solution- 0.5 g/l; TAP-H solution- 1.5 g/l, p.o.; for 10 weeks |
Genetically type
2 diabetic model (KK-Ay mice) |
Reduced serum glucose
levels, total cholesterol and triglyceride levels; Significant decrease in plasma lipoperoxide level |
| 35. | Tremella
fuciformis Berk. (Tremellaceae) Snow fungus, Silver ear fungus, White jelly mushroom |
Glucuronoxylom
annan (AC) from the fruiting bodies |
Oral
administrat- ions of the AC solution |
Normal and
STZ- induced diabetic mice |
Significant dose-
dependent hypo-glycemic activity |
| Exopolysacch-
arides (EPS) produced by submerged mycelial culture |
(0.75 g/l)
200 mg/kg for 52 days, p.o. |
ob/ob Mice | Hypoglycemic effects and
improved insulin sensitivity possibly through regulating PPAR- γ mediated lipid metabolism |
||
| 36. | Tremella
mesenterica (Schaeff.) Retz. (Tremellaceae) Yellow brain mushroom, Golden jelly fungus, Yellow trembler, Witches’ butter |
Tremellastin,
containing 40- 45% acidic polysaccharide glucuronoxy- lomannan, obtained by alcoholic precipitation of culture broth after submerged cultivation |
100 mg/kg
and 500 mg/kg, p.o. |
STZ-induced
hyperglyc-emic mice |
Statistically significant
and dose-dependent reduction of intrinsic blood glucose levels as well as significantly decreased triglyceride levels |
| Fruiting bodies | – | STZ-induced | Significant reduction in |
| containing acidic
heteropolysa- ccharide and several sugars including glucose |
type 1 diabetic
rats and nicotinamide and STZ- induced prediabetic impared glucose tolerant rats |
elevated blood glucose
levels |
|||
| 37. | Wolfiporia extensa
(Peck) Ginns (Polyporaceae) Pine-tree rotting mushroom |
Crude extract
containing dehydro- tumulosic acid, dehydro- trametenolic acid and pachymic acid |
– | STZ-induced
diabetic mice |
Insulin sensitizer activity |
Table 2: Clinical studies carried out with mushrooms for management of DM.
| S.no. | Biological source | Extract/
Fraction/ Isolate |
Dose | Type of trial | Observations |
| 1. | Agaricus sylvaticus
Schaeff. (Agaricaceae) Sun Mushroom |
Fruiting bodies | 30mg/kg;
Dietary supplementation |
Random-
ized, double- blind, placebo- controlled clinical trial on 56 patients with colorectal cancer |
Significant
reduction of fasting plasma glucose,total cholesterol, creatinine, aspartate aminotransf- erase,alanine aminotransf-erase, systolic blood pressure |
| 2. | Grifola frondosa
(Dicks.) Gray (Fomitopsidaceae) Hen of the woods, Maitake |
Grifola
frondosa polysacchande caplets (MFCs) containing active SX- fraction.. |
– | 5 patients
with type 2 diabetes |
Improved glycemic
levels. One patient showed complete glycemic control with MFCs; whereas others showed over 30% decline in their serum glucose levels with MFCs in 2 to 4 weeks |
| 3. | Pleurotus ostreatus
(Jacq.) P. Kumm. (Pleurotaceae) Oyster mushroom |
Powdered
fruiting bodies |
Dietary supplem-
entation |
120 patients
with type 2 diabetes |
Significant
association between mushroom supplement-ation and gradual reduction in hyperglycemia in type 2 diabetic subjects |
Conclusion
The review demonstrates that agaricus bisporus have a great potential for the production of useful bioactive metabolites and those they are a prolific resources for drugs. The responsible bioactive compounds belong to several chemical groups. Agaricus bisporus possess a high variety of bioactive compounds, and therefore of pharmacological effects.
Acknowledgement
The authors wish to express their sincere gratitude to Department of Pharmacy, Kailash Institute of Pharmacy & Management, GIDA, Gorakhpur, UP, India, for providing necessary facilities to carry out this research work.
References
- Amandip Kaur, Gurpaul Singh Dhingra, Richa Shri; Antidiabetic potential of mushrooms. Asian J. Pharma. Res. 5(2);111-125. 2015
- Dilani D. De Silva & Sylvie Rapior & Kevin D. Hyde & Ali H. Bahkali, Medicinal mushrooms in prevention and control of diabetes mellitus Fungal Diversity. 56:1–29. 2012.
- V. Venkatash gobi1, Bio-Chemical Extraction of Active Compounds of Agaricus (Mushrooms) and it’s Antioxidant Activity. IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 14, Issue 9 Ver. V (Sep. 2015), PP 36-39. 2015.
- Ulrike Lindequist, Timo H. J. Niedermeyer, and Wolf-Dieter Jülich, The Pharmacological Potential of Mushrooms, Evid Based Complement Alternat Med. 2(3):285–299. 2005.
- Ram Chandra, V.N Pandey & H.B singh, Extract of white button mushroom(Agaricus bisporus)for bio-medicinal molecules; CIB Tech Journal of Pharmaceutical sciences. 1(1); 9-11. 2012.
- Mariappansenthi kumar, Vinayagam srividhya, Durai mahalakshmi. Phytochemical screening of bioactive compounds from pleurotus ostreatus kumm., An wild edible mushroom, wjpr. 4(05),1603-1618. 2015.
- Dhamodharam G. & Mirunalini S, A Novel medicinal characterizatio of Agaricus bisporus; Pharmacologyonline. 2: 456-463. 2010.
- Nasiri,B.Ghiassi Tarzi. Annals of Biological Research. 3(12):5677-5680. 2012.
- Katherine M.Phillips, Ronald L.Horst, Nicholas J.Koszewski,Ryan R. Simon. Vitamin D4 in mushrooms. 7(8) Plos one. 2012.
- Amandip Kaur, Gurpaul Singh Dhingra, Richa Shri; Antidiabetic potential of mushrooms. Asian J. Pharma. Res. 5(2);111-125. 2015
- Liu J1, Jia L, Kan J, Jin CH,In vitro and in vivo antioxidant activity of ethanolic extract of white button mushroom (Agaricus bisporus).,Food Chem Toxicol. 51:310-6. 2013.
- Bożena Muszyńska1,*, Katarzyna Kała1, Jacek Rojowski2, Agata Grzywacz1, Włodz imierz Opoka Composition and Biological Properties of Agaricus bisporus Fruiting Bodies – a Review, Pol. J. Food Nutr. Sci. 67(3); 206-9. 2017.