Palladium-based materials possess unique chemical and physical properties, making them indispensable across multiple industries, including instrumentation, chemical manufacturing, jewelry, dentistry, and medicine¹. Owing to palladium’s widespread applications, there is a critical need for rapid and accurate methods to determine Pd(II) in real samples.
Several analytical techniques—atomic absorption spectrometry²˒³, spectrophotometry⁴˒⁵, voltammetry⁶˒⁷, ICP-OES⁸˒⁹, and ICP-MS¹⁰—have been employed for Pd(II) analysis. Among them, solvent extraction has gained prominence as an effective separation tool due to its simplicity, speed, and versatility, especially at trace levels.
Extractive spectrophotometric methods for palladium have utilized reagents such as hydrazones, thiosemicarbazones, oximes, and thioureas. Thiosemicarbazones, in particular, are notable for their strong reactivity with metal ions and their ability to form stable, colored complexes. They also exhibit various pharmacological properties including antiparasitic¹¹˒¹², antiviral¹³˒¹⁴, antimicrobial¹⁵, antifungal¹⁶˒¹⁷, and antitumor¹⁸ activities.
Despite many methods using organic reagents for Pd(II) extraction, challenges remain with respect to speed, sensitivity, and selectivity. This study presents a highly selective, sensitive, and rapid spectrophotometric method for Pd(II) determination using CSTSC as a reagent, offering advantages over previously reported methods (Table 1).
Table 1. Comparison of the Proposed Method with Reported Methods for Pd(II) Determination
| Reagent | pH / Acid/Base | λ (nm) | Beer’s Range (µg mL⁻¹) | Molar Absorptivity (L mol⁻¹ cm⁻¹) | Molar Ratio | Remarks | Ref. |
|---|---|---|---|---|---|---|---|
| 3-Phenoxybenzaldoxime | 4.0 | 435 | 0.4 – 4.0 | 2.434 × 10³ | NR | Less sensitive | 19 |
| 4-(N,N-diethylamino)benzaldehyde thiosemicarbazone | 3.0 | 408 | 0.36 – 3.24 | 3.337 × 10⁴ | 1:2 | Narrow Beer’s range | 20 |
| Isonitroso p-nitroacetophenone thiosemicarbazone | 0.0 – 4.0 | 410 | 5.0 – 80 | 9.10 × 10² | 1:2 | Less sensitive | 21 |
| p-[N,N-bis(2-chloroethyl)amino]benzaldehyde thiosemicarbazone | 0.2 M HCl | 395 | 0.48 – 2.40 | 4.05 × 10⁴ | 1:2 | Narrow Beer’s range | 22 |
| o-Methoxyphenyl thiourea | 1.0 HCl | 325 | 0 – 15 | 3.38 × 10³ | 1:1 | Absorbance in UV region | 23 |
| 3-Methoxysalicylaldehyde-4-hydroxybenzoyl hydrazone | 4.5 | 412 | 0.287 – 4.256 | 1.03 × 10³ | NR | Less sensitive, requires surfactant | 24 |
| o-Methylphenyl thiourea | 0.8 M HCl | 340 | 0 – 12.5 | 2.85 × 10³ | 1:1 | Absorbance in UV region | 25 |
| 4-(4′-fluorobenzylideneimino)-3-methyl-5-mercapto-1,2,4-triazole | 0.6 – 2.0 HCl | 390 | 4.12 – 17.5 | 5.404 × 10³ | 1:1 | Less sensitive | 26 |
| 3-Hydroxy-2-[2′-(5′-methylthienyl)]-4-oxo-4H-1-benzopyran | 0.5 M NaHCO₃ | 418 | 0 – 1.3 | 6.597 × 10⁴ | 1:1 | Narrow Beer’s range | 27 |
| 6-Chloro-3-hydroxy-7-methyl-2-(2′-thienyl)-4-oxo-4H-1-benzopyran | 0.5 M NaHCO₃ | 415–426 | 0 – 2.6 | 6.173 × 10⁴ | 1:1 | Narrow Beer’s range | 28 |
| 5-Chlorosalicylaldehyde thiosemicarbazone (PM) | 4.0 | 400 | 0.5 – 9.0 | 1.072 × 10⁴ | 1:2 | Rapid, highly sensitive and selective | PM |
Notes:
- NR: Not reported
- PM: Present method
Materials and Methods
Instruments and Chemicals
Absorbance measurements were performed using a Jasco V-730 UV-VIS spectrophotometer with a 1.0 cm quartz cell. pH was adjusted using an Equiptronics pH meter. Weighing was carried out with a Contech electronic balance (CA 64). All chemicals used were of analytical reagent grade. Freshly prepared doubly distilled water was used throughout. A 1000 ppm stock solution of Pd(II) was prepared by dissolving PdCl₂ in doubly distilled water acidified with HCl. This stock solution was standardized gravimetrically using dimethylglyoxime²⁹ and diluted daily for use. Buffers at pH 3.6–4.4 were prepared by mixing 0.2 M KHP with 0.2 M HCl or NaOH.
Solutions of Foreign Ions
Stock solutions of anions and cations were prepared by dissolving appropriate salts or acids in distilled water, adjusting to known volumes.
Synthesis of CSTSC
Equimolar amounts of 5-chlorosalicylaldehyde (1.567 g) in 25 mL ethanol and thiosemicarbazide (0.911 g) in aqueous ethanol were refluxed for 5 hours. The mixture was poured into ice to precipitate CSTSC, which was filtered, washed with water, recrystallized from ethanol, and dried. A 0.1% CSTSC solution was prepared from the recrystallized product.
Procedure
To a 10 mL volumetric flask, solutions containing 5.0–90.0 μg mL⁻¹ Pd(II), 1.0 mL of 0.1% CSTSC in methanol, and 4.0 mL phthalate buffer (pH 4.0) were combined. The volume was brought to 10 mL with distilled water. This solution was transferred to a separating funnel and equilibrated with 10 mL butyl acetate. After phase separation, the organic layer was dried over anhydrous sodium sulfate. The absorbance of the organic phase was measured at 400 nm against a reagent blank.
Results and Discussion
Absorption Spectra
Upon complexation with CSTSC, Pd(II) formed a yellow complex that could be quantitatively extracted into butyl acetate at pH 3.6–4.4. The absorption spectra (Fig. 1) showed two maxima at 375 nm and 400 nm, with 400 nm chosen due to minimal reagent absorbance interference.
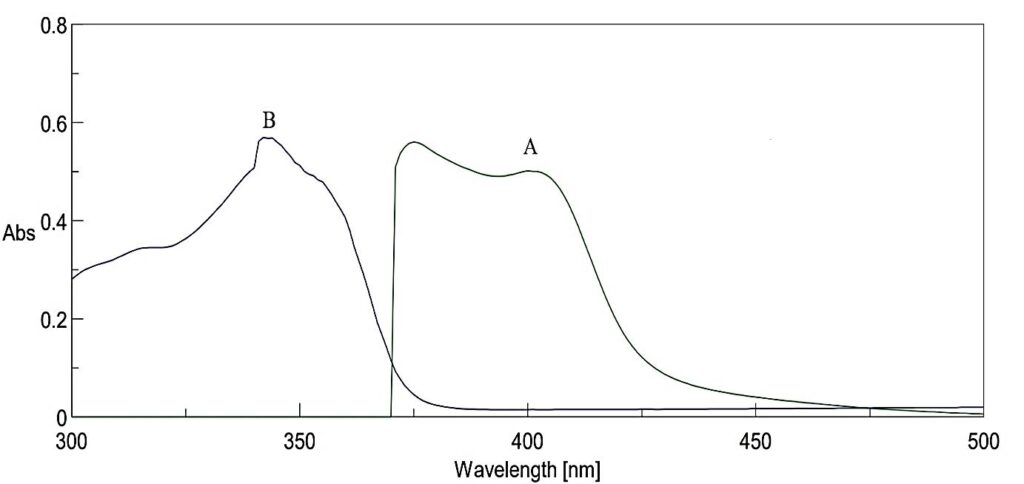
Fig.1: A – Pd-CSTSC Complex absorption spectra vs. CSTSC blank
B – CSTSC absorption spectra vs. butyl acetate blank
pH Optimization
Extraction experiments across pH 1–10 revealed maximum complexation and absorbance within pH 3.6–4.4, with pH 4.0 selected for further studies.
Effect of Solvent
A range of organic solvents was evaluated for extraction efficiency. The order of extraction efficiency was: butyl acetate > n-butanol > ethyl acetate > isoamyl alcohol > cyclohexanone > toluene > benzene > chloroform > carbon tetrachloride > xylene > hexane. Butyl acetate provided ~99.9% extraction and was used for all extractions.
Reagent Concentration
Optimal color development was achieved with a threefold molar excess of CSTSC, beyond which absorbance plateaued (Fig. 2). Therefore, 1.0 mL of 0.1% CSTSC was used in all analyses.
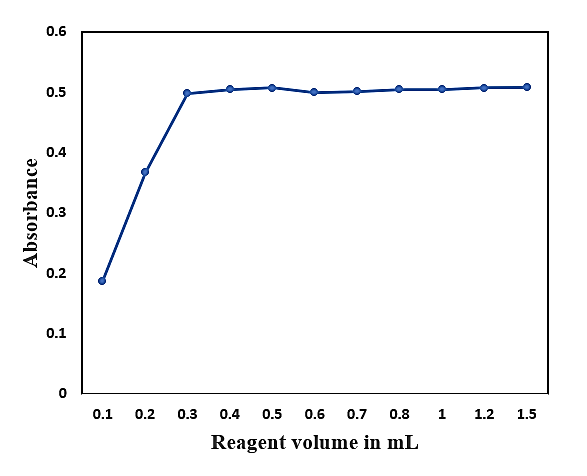
Fig.2: The impact of concentration of reagent on Pd(II)-CSTSC complex absorbance
Equilibration Time and Complex Stability
Complete extraction was achieved within 30 seconds. The extracted complex remained stable for over 96 hours without significant absorbance change.
Effect of Salting-Out Agents
Addition of salting-out agents (e.g., ammonium sulfate, sodium chloride) did not influence absorbance, indicating extraction efficiency was unaffected.
Beer’s Law Range and Sensitivity
The Pd(II)-CSTSC complex obeyed Beer’s law in the range 0.5–9.0 μg mL⁻¹ at 400 nm (Fig. 3). Sandell’s sensitivity was 9.92 × 10⁻³ μg cm⁻², and molar absorptivity was 1.072 × 10⁴ L mol⁻¹ cm⁻¹.
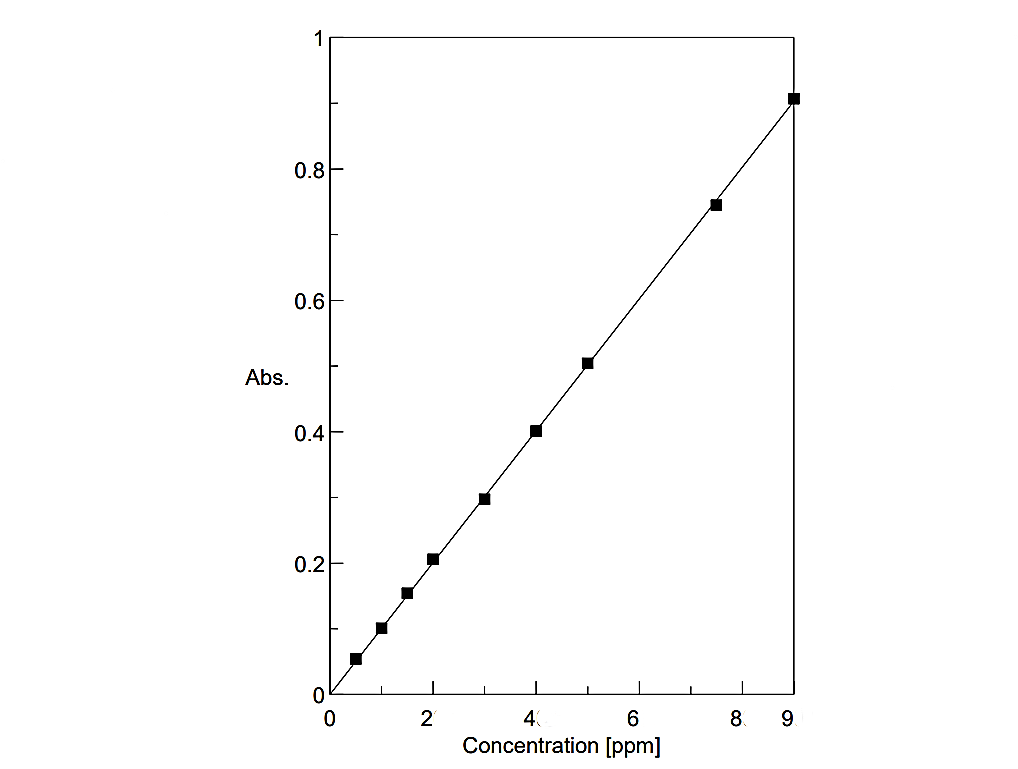
Fig.3: Calibration plot of Pd(II)-CSTSC complex
Complex Composition
Using Job’s method and mole ratio plots (Figs. 4 and 5), the complex stoichiometry was established as 1:2 (Pd:CSTSC).
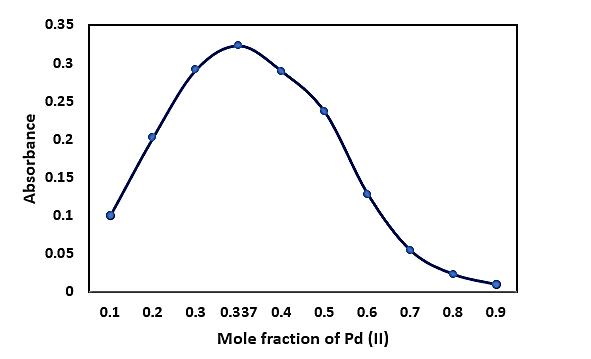
Fig.4: Job’s Continuous Variation Method of the Complex of Pd(II)-CSTSC
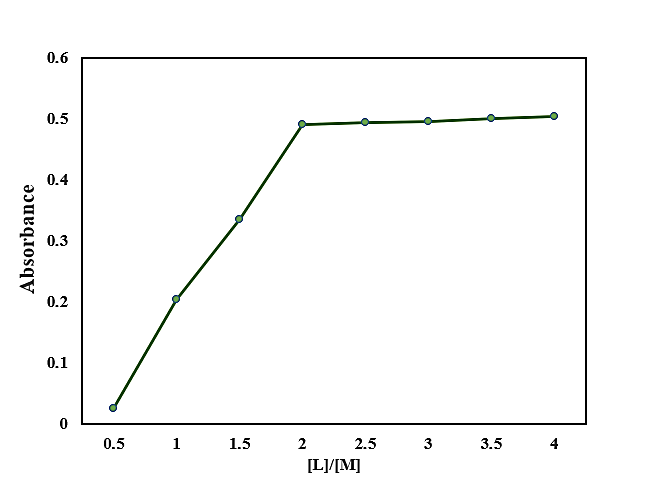
Fig.5: Mole Ratio Method of the Complex of Pd(II)-CSTSC
Interference Study
The tolerance limits for various ions are presented in Table 2. Most common ions did not interfere, but Co(II), Pd(II), Ru(III), Ag(I), and Cu(II) caused interference, which could be mitigated with suitable masking agents.
Precision and Accuracy
Ten replicate measurements of 5.0 μg mL⁻¹ Pd(II) yielded a standard deviation of 0.0288 and relative standard deviation of 0.58%, indicating high precision (95% confidence interval: 4.988 ± 0.01783).
Applications
Synthetic Mixtures
Pd(II) was determined in synthetic mixtures with various interfering metals. Results (Table 3) demonstrated accurate recovery.
Water Samples
Spiked tap and well water samples were digested and analyzed with excellent recoveries (Table 3).
Palladium Complexes
Synthesized palladium dimethylglyoxime and thiosemicarbazide complexes were digested and analyzed, yielding results consistent with expected Pd content (Table 4).
Conclusion
This method provides a rapid, sensitive, and selective approach for the spectrophotometric determination of Pd(II) in microgram quantities. The procedure is simple, effective even in the presence of diverse ions, and applicable to real samples including water, synthetic mixtures, and palladium complexes.
Acknowledgment
This research received no specific funding from public, commercial, or not-for-profit agencies.
Conflict of Interest
The authors declare no conflicts of interest.
References
- Encyclopaedia of Environmental Health, Elsevier B. V, 2011, 307;
- Sivrikaya, S.; Karslı, B.; Imamoglu, M; Int. J. Environ. Res., 2017, 11, 579;
- Eskina, V.; Dalnova, O.; Filatova, D.; Baranovskaya, V.; Karpov, Y.; Spectrochim. Acta B Atom. Spectrosc., 2020, 165,105784;
- Kumar, A.; Gupta, S.; Barhate, V.; Orien. J. Chem., 2010, 26, 1085;
- Reddy, K.; Kumar, J.; Ramachandraiah, C.; Reddy, S.; Reddy, A.; Environ. Monit. Assess., 2008, 136, 337;
- Sadowska, M.; Kińska, K.; Kowalska, J.; Ostręga, B.; Microchem. J., 2020, 154, 104557;
- Rydchuk, P.; Labyk, O.; Oleksiv, L.; Tymoshuk, O.; Chaban, T., Chem. and Chem. Tech., 2021, 15, 319;
- Merusomayajula, K.; Tirukkovalluri, S.; Kommula, R.; Chakkirala, S.; Vundavilli, J.; Kottapalli, P., Futur. J. Pharm. Sci., 2021, 7, 45;
- Souza, E.; Amaral, C.; Nagata, N.; Grassi, M., Microchem. J., 2020, 152, 104309;
- Leśniewska, B.; Arciszewska, Ż.; Wawrzyńczak, A.; Jarmolińska, S.; Nowak, I.; Godlewska-Żyłkiewicz, B., Talanta, 2020, 217,121004 ;
- Chellan, P.; Stringer, T.; Shokar, A.; Dornbush, P.; Vazquez-Anaya, G.; Land, K.; Chibale, K.; Smith, G.; J. of Inorg. Biochem., 2011, 105, 1562;
- Chellan, P.; Land, K.; Shokar, A.; Au, A.; Hwan An, S.; Clavel, C.; Dyson, P.; Kock, C.; Smith, P.; Chibale, K.; Smith,G., Organometallics, 2012, 31, 5791;
- Padmanabhan, P.; Khaleefathullah, S.; Kaveri, K.; Palani, G.; Ramanathan, G.; Thennarasu, S.; Tirichurapalli, S.; J Med Virol., 2017, 89, 546;
- İyidoğan, A. ; Taşdemir, D.; Emre, E.; Balzarini, J.; Eur. J. Med. Chem., 2011, 46, 5616;
- Tamayo, L.; Burgos, A.; Brandão, P.; Phosp., Sul., and Sili. and the Rel. Ele., 2014, 189, 52;
- Paiva, R.; Kneipp, L.; Goular, C.; Albuquerque, M.; Echevarria, A., Agri. Sci., 2014, 38, 6;
- Bajaj, K.; Buchanan, R.; Grapperhaus, C.; J. of Inorg. Biochem., 2021; 225, 111620;
- Zhang, B.; Luo, H.; Xu, Q.; Lin, L.; Zhang, B., Oncotarget., 2017, 8, 13620;
- Lokhande, R.; Nemade, H.; Chaudhary, A.; Hundiwale, D., Asian J. of Chem., 2001, 13, 596.
- Parameshwara, P.; Karthikeyan, J.; Shetty, A.; Shetty, P., Annali di Chim., 2007, 97, 1097.
- Barhate, V.; Madan, P.; Gupta, A.; Mandhare, D., Orient. J. Chem., 2009, 25, 731.
- Kuchekar, S.; Naval, R.; Han, S., S. Afr. J. Chem., 2014, 67, 226.
- Karthikeyan, J.; Parameshwara, P.; Shetty, A., Environ Monit Assess, 2011, 173, 569;
- Rao, M.; Chandrasekhar, K., Eur. J. Applied Eng. Scient. Res., 2012, 1, 48.
- Shelar, Y.; Aher, H.; Kuchekar, S.; Han, S., Bulg. Chem. Comm., 2012, 45, 172.
- Shaikh, A.; Barache, U.; Lokhande, T.; Kamble, G.; Anuse, M.; Gaikwad, S., Rasayan J. Chem, 2017, 10, 967;
- Kaur, N.; Agnihotri, N.; Agnihotri, R., Vietnam J. Chem., 2019, 57, 686;
- Kaur, N.; Agnihotri, N.; Berar, U., Asian J. Chem., 2020, 32, 1597.
- Vogel’s textbook of quantitative chemical analysis,Longman, Green, 1961, 463.
- D. Mahadevappa, B. Gowda, A. Murthy, Curr. Sci., 1976, 45, 161.

