Biguanide and its derivatives have long been utilized as drugs in medical science. Metformin is commonly prescribed as an oral hypoglycemic agent for patients with type 2 diabetes. Similarly, proguanil is used as an antimalarial drug; phenyl biguanide acts as a 5-HT3 receptor agonist; and phenylaminopropyl biguanide serves as a disinfectant and antimalarial agent. Biguanide itself is formed through the condensation of two guanidine molecules, accompanied by the elimination of one molecule of ammonia. This process is illustrated in Figure 1 below:
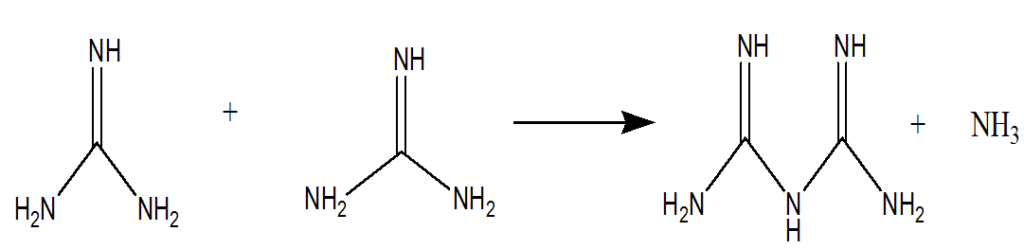
Fig 1. Condensation reaction of guanidine molecules to form biguanide with elimination of ammonia
Guanidine hydrochloride is used and fused at 180–185 °C. Therefore, biguanide is also known as guanylguanidine. Piperazinedibiguanidine is a tetradentate ligand, similar to ethylenedibiguanide. The structures of both compounds are shown in Figure 2.
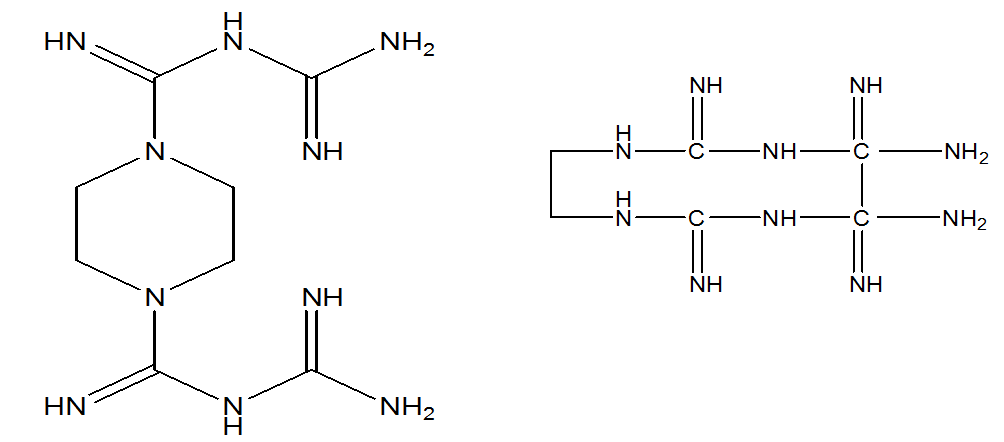
Fig 2 structure of (a) piperazinedibiguanide and (b) ethylene dibiguanide
Coordination complexes of certain thiocarbamoyl dihydropyrazoles with copper and nickel ions exhibit notable antifungal activity against various Candida strains. Analysis has revealed that copper complexes demonstrate greater activity than their nickel counterparts. Piperazine, classified as an antihelminthic, is used medically to treat infections caused by common roundworms (ascariasis) and pinworms (enterobiasis). This compound was reacted with dicyandiamide to produce its biguanide derivative. Although biguanide and its derivatives complexed with transition metals have been studied extensively, investigations into the properties of this specific ligand remain limited, both in terms of physicochemical and biochemical characteristics.
In the present work, the authors focus on the magnetic properties and antifungal activity of piperazinedibiguanide complexes. To achieve this, the ligand was first synthesized using established methods, then complexed with Cu(II) ions through reactions with their salts. Following complexation, magnetic measurements were conducted, and the antifungal activity of the resulting complexes was evaluated and discussed in this research report. The fungi tested were Aspergillus niger and Aspergillus versicolor.
Biguanide iridium(III) complexes have demonstrated significant antifungal efficacy against Candida albicans and Candida neoformans, with minimum inhibitory concentrations (MICs) reaching the nanomolar range—as low as 0.25 µg/ml.
Complexes of Cr(III), Ni(II), and Cu(II) with the p-phenylenedibiguanide ligand have shown more than 80% inhibition of fungal growth in A. niger and A. versicolor at 400 µg/ml, compared to controls with the free ligand on both PDA and SDA media.
Cobalt(III) complexes containing a mixed ligand with one biguanide moiety also exhibited more than 70% inhibition of A. niger and over 80% inhibition of A. versicolor at 400 µg/ml in both PDA and SDA media.
Bis(phenylbiguanidium)nickel(II) chloride and α-nickelphenylbiguanidine(II) complexes, which also feature biguanide ligands, displayed antifungal activity against A. niger and A. versicolor, achieving over 80% inhibition at 400 µg/ml in both PDA and SDA media.
Complexes of copper piperazinedibiguanide chloride and nickel piperazinedibiguanide chloride showed complete (100%) inhibition of A. niger and Penicillium digitatum at 1000 µg/ml in both PDA and SDA media.
This review highlights the significant potential for further research into the biochemical activity of biguanide derivatives as ligands complexed with metal ions. The two fungi studied by the authors are detailed below:
Aspergillus niger is a common species within the genus Aspergillus. It is known to cause black mold on fruits and vegetables, is prevalent in soil, and is frequently found in indoor environments.
Aspergillus versicolor is widely distributed in nature, typically found on substrates exposed to moisture or slow decomposition, especially in cold regions and cultivated soils, though it is rarely found in forests. A. versicolor is both toxic and pathogenic to humans and animals; the aflatoxins it produces are implicated in the development of various cancers.
The primary aim of this study was to assess the antifungal activity of these complexes. If found effective, they could serve as antifungal agents in various everyday products and potentially provide alternative treatments against microbes developing resistance to conventional antifungal compounds.
Materials and methods
Preparation of the Ligand and Its Complexes
The ligand and its complexes were synthesized following previously reported methods, as detailed below:
Piperazinedibiguanide sulfate (C₈H₁₈N₁₀•2H₂SO₄•1.5H₂O):
To prepare this compound, 4.3 g of piperazine was heated with 8.4 g of dicyandiamide in 75 ml of water in a conical flask placed on a water bath for 3 hours. During heating, 5 ml of an aqueous CuSO₄ solution was added in small portions every 20 minutes. The resulting mixture was treated with an aqueous solution of 2.8 g sodium hydroxide, which caused the mixture to turn reddish-purple. After cooling, the mixture was filtered to separate the purple residue. This residue was decomposed with dilute sulfuric acid (1:3), and upon cooling, white piperazinedibiguanide sulfate began to crystallize. The crystals were filtered and washed with cold water until free of copper ions. The white residue was dissolved in an ammonia solution containing a small amount of sodium hydroxide, then acidified with dilute sulfuric acid (1:3). On cooling, shiny white crystals of piperazinedibiguanide sulfate formed. These were filtered, washed with cold water, and air-dried.
Copper piperazinedibiguanide hydroxide (Cu Pip(BigH)₂₂•3H₂O):
This complex was prepared by adding an ammoniacal solution of 4.7 g piperazinedibiguanide sulfate to an ammoniacal solution of 1.6 g cupric sulfate with constant stirring. A reddish-violet precipitate formed immediately, which was filtered and washed with cold water until sulfate ions were completely removed. The product was dried in a desiccator until a constant weight was reached.
Copper piperazinedibiguanide chloride ([Cu Pip(BigH)₂]Cl₂•4H₂O):
To prepare this compound, the copper piperazinedibiguanide hydroxide was heated with aqueous ammonium chloride on a water bath. Heating was stopped when ammonia evolution ceased, leading to the precipitation of a reddish-violet chloride complex. It was washed with cold distilled water and air-dried. The compound showed limited solubility in hot water and was insoluble in alcohol.
Copper piperazinedibiguanide sulfate ([Cu Pip(BigH)₂]SO₄•5.5H₂O):
This complex was synthesized similarly to the chloride by treating the hydroxide base with an aqueous solution of ammonium sulfate. The resulting violet complex was insoluble in water and alcohol.
Copper piperazinedibiguanide nitrate (Cu Pip(BigH)₂₂•H₂O):
This complex was obtained by treating the hydroxide base with a solution of ammonium nitrate. The resulting red powder was insoluble in water and alcohol.
Antifungal Activity
Antifungal activity was evaluated by inoculating the prepared fungal strains onto PDA (Potato Dextrose Agar) and SDA (Sabouraud Dextrose Agar) media. Both media were autoclaved prior to use, and the complex solution and medium were mixed at a ratio of 1:10. The experiments were conducted in triplicate for each Petri dish at specified concentrations. Mycelial inhibition was determined using the disc dilution method. Preparation of the media was as follows:
Preparation of Potato Dextrose Agar (PDA):
Potato tubers (200 g) were peeled, chopped into small pieces, and boiled in 100 ml of distilled water for 20 minutes. The mixture was filtered through muslin cloth. To the filtrate, 20 g dextrose, 15 g agar, and 2 g peptone were added, and the solution was gently heated. The volume was adjusted to 1 liter, maintaining a pH of 5.6 using 1N HCl or NaOH. The medium was stored in an Erlenmeyer flask and autoclaved at 121°C for 20 minutes before use.
Preparation of Sabouraud Dextrose Agar (SDA):
For SDA, ingredients including water, dextrose, agar, peptone, and antibiotics were combined. When using a premix, about 70 g of the mix was dissolved in 1 liter of water and heated until the agar was completely dissolved. The pH was adjusted to 5.5 using 1M hydrochloric acid. The medium was then autoclaved and stored at room temperature. This medium was suitable for inoculation with fungal spores, and mycelial inhibition was assessed using standard methods.
Following media preparation, pure fungal cultures were isolated using the Warcup method. These cultures were inoculated into fresh Petri dishes containing the prepared media for further isolation and identification. The fungi were identified microscopically, and additional testing was carried out in the UV chamber.
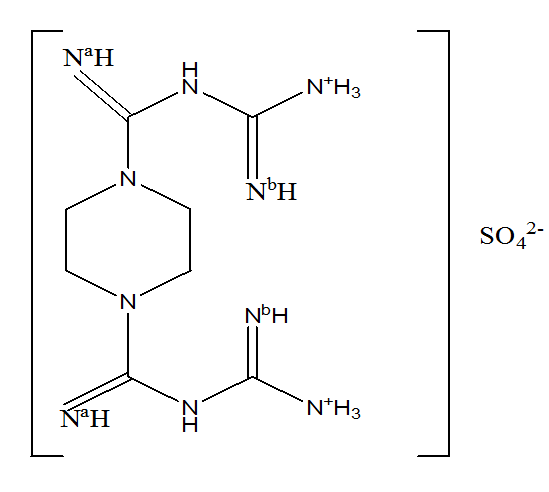
Figure 3. showing atoms of Piperazinedibiguanide sulphate coordinating to metal ions to form complexes
Results
Piperazinedibiguanide sulphate is a quadridentate chelating ligand, and its complexes with divalent metals are well-known. The ligand coordinates through the nitrogen atoms Na and Nb of both biguanide groups, forming typical six-membered rings with the Cu(II) ion (see Figure 3). UV spectral analysis confirms the formation of a bond between the metal ion and the ligand’s nitrogen atoms. Elemental analysis results are presented in Table 1. Magnetic measurements revealed that all copper(II) complexes with the piperazinedibiguanide ligand exhibit paramagnetism (Table 2), suggesting that the complexes adopt a planar structure.
The synthesized complexes were dissolved in appropriate solvents at various concentrations and tested against fungi isolated under UV light. These solutions demonstrated antifungal activity against two ascomycetes, Aspergillus niger and Aspergillus versicolor. When cultured on PDA and SDA media, both fungi showed complete (100%) inhibition of growth at complex concentrations between 500–800 μg/ml. Interestingly, one piperazinedibiguanide-Cu(II) complex achieved 100% inhibition at a lower concentration of 400 μg/ml. Micelle inhibition was subsequently quantified, and the data are summarized in Tables 3 and 4.
| Elemental Analysis in % | ||||||
| Compound | N | S | Cu | Cl | water | |
| Piperazinedibiguanide | Found | 29 | 13.97 | 7.6 | ||
| C8H18N10•2H2 SO4•1.5H2O | Calculated | 29.35 | 13.41 | 7.82 | ||
| Copper piperazinedibiguanide hydroxide | Found | 34.96 | 15.4 | 21.95 | ||
| [Cu Pip(BigH)2](OH)2•3H2O | Calculated | 34.5 | 15.6 | 22.10 | ||
| Copper piperazinedibiguanide chloride | Found | 14.09 | 15.78 | 15.30 | ||
| [Cu Pip(BigH)2]Cl2•4H2O | Calculated | 13.7 | 15.04 | 15.60 | ||
| Copper piperazinedibiguanide sulphate | Found | 6.47 | 12.98 | 19.12 | ||
| [Cu Pip(BigH)2]SO4•5.5H2O | Calculated | 6.2 | 12.4 | 19.31 | ||
| Copper piperazinedibiguanide nitrate | Found | 13.2 | 4.01 | |||
| [Cu Pip (BigH) 2](NO3)2•H2O | Calculated | 13.8 | 3.9 | |||
Table 1. Elemental analysis of different element in ligand and metal complexes
| Magnetic behaviour of complexes | |
| Compound | magnetic behaviour |
| Copper piperazinedibiguanide hydroxide | paramagnetic |
| Copper piperazine dibiguanide chloride | paramagnetic |
| Copper piperazinedibiguanide sulphate | paramagnetic |
| Copper piperazinedibiguanide nitrate | paramagnetic |
Table 2. Magnetic behavior of complexes
| Inhibition Percentage of growth of fungus Aspergillus niger at indicated dose | |||
| Metal complex | Concentration | Inhibition % of fungal growth | |
| PDA | SDA | ||
| Copper piperazinedibiguanide hydroxide | 400 μg/ml | 96.97% | 94.30% |
| 200 μg/ml | 78.49% | 72.14% | |
| 100 μg/ml | 71.57% | 69.91% | |
| Copper piperazine dibiguanide chloride | 400 μg/ml | 95.80% | 93.11% |
| 200 μg/ml | 87.25% | 79.59% | |
| 100 μg/ml | 78.52% | 74.45% | |
| Copper piperazinedibiguanide sulphate | 400 μg/ml | 97.15% | 94.63% |
| 200 μg/ml | 87.21% | 84.08% | |
| 100 μg/ml | 79.70% | 77.07% | |
| Copper piperazinedibiguanide nitrate | 400 μg/ml | 93.01% | 89.09% |
| 200 μg/ml | 85.72% | 81.37% | |
| 100 μg/ml | 75.36% | 72.25% | |
Table 3. Inhibition Percentageof mycelial growth of A. niger in PDA and SDA
| Inhibition Percentage of growth of fungus Aspergillus Versicolor at indicated dose | |||
| Metal complex | Concentration | Inhibition % of fungal growth | |
| PDA | SDA | ||
| Copper piperazinedibiguanide hydroxide | 400 μg/ml | 100% | 99.10% |
| 200 μg/ml | 88.89% | 86.78% | |
| 100 μg/ml | 79.40% | 77.40% | |
| Copper piperazine dibiguanide chloride | 400 μg/ml | 98.50% | 95.20% |
| 200 μg/ml | 87.28% | 79.27% | |
| 100 μg/ml | 69.51% | 63.31% | |
| Copper piperazinedibiguanide sulphate | 400 μg/ml | 95.00% | 90.82% |
| 200 μg/ml | 82.50% | 79.75% | |
| 100 μg/ml | 69.57% | 60.91% | |
| Copper piperazinedibiguanide nitrate | 400 μg/ml | 92.31% | 87.50% |
| 200 μg/ml | 78.73% | 70.58% | |
| 100 μg/ml | 62.72% | 53.41% | |
Table 4. Percentage inhibition of mycelial growth of A. versicolor in PDA and SDA
Discussion and Interpretation
Elemental analyses of the ligands and their complexes are presented in Table I, showing good agreement between the experimental and theoretical values. The UV-Visible spectral analysis revealed peaks at 370 nm, 240 nm, and 460 nm, which correspond to the following electronic transitions, confirming the coordination of the metal ion with the nitrogen atoms of the ligand:
- 370 nm → Charge Transfer (CT)
- 240 nm → Charge Transfer (CT)
- 460 nm → 2B1g→2B2g^2B_{1g} \rightarrow ^2B_{2g}2B1g→2B2g
Table III shows that the compounds exhibit paramagnetic behavior. Data presented in Tables III and IV are further analyzed and illustrated in eight statistical graph plots demonstrating the biochemical activities. Graphs 1a to 1d indicate that the complexes were more effective against A. niger in PDA medium, where they significantly inhibited fungal growth compared to SDA medium. Similarly, graphs 2a to 2d, which represent the activity against A. versicolor, show that growth inhibition was greater in PDA medium. Notably, for copper piperazinedibiguanide hydroxide, antifungal activity was comparable in both PDA and SDA media, indicating that the compound is equally effective in both.
Conclusion
Based on the MIC results and the discussions above, it can be concluded that all Cu(II) complexes of piperazinedibiguanide are paramagnetic (Table 2) and adopt square planar geometry. Regarding antifungal activity against A. niger (Table 3), copper complexes with piperazinedibiguanide ligand in PDA medium inhibited fungal growth by over 90%, while in SDA medium the inhibition exceeded 89% at a concentration of 400 μg/ml. Similarly, for A. versicolor (Table 4), all complexes inhibited fungal growth by more than 90% in PDA medium and over 80% in SDA medium at the same concentration. Additionally, copper piperazinedibiguanide hydroxide demonstrated complete (100%) inhibition of A. versicolor growth in PDA medium and 99.10% inhibition in SDA medium at 400 μg/ml.
Conflict of Interest
The author declares no financial or non-financial conflict of interest related to the content of this manuscript.
References
- Abdel-Rahman, L. H.; Abu-Dief, A. M.; Ismael, M.; Mohamed, G. G. “Ni(II), Pd(II) and Pt(II) complexes with tridentate hydrazone ligands: Spectral, thermal, molecular modeling and antimicrobial studies.” Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2015, 138, 802–814.
- Shahabadi, N.; Kashanian, S.; Darabi, F. “DNA binding and spectral properties of Cu(II) complex containing dinitrogen Schiff base ligand.” Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2010, 75(2), 422–426.
- Rezaeivala, M.; Keypour, H.; Haghighi, M. G.; Heidari, M. “Nickel(II) and copper(II) complexes with tetradentate Schiff base ligands: Synthesis, characterization and antibacterial studies.” Polyhedron 2011, 30(4), 641–648.
- Cox, D. N.; Marley, N. J.; Smith, M. A.; Jenkins, H. D. “Magnetic susceptibility measurements of transition metal complexes.” Journal of Chemical Education 2013, 90(7), 951–954.
- Tiwari, A.; Prasad, S. R.; Rai, V. K. “Synthesis, characterization, thermal, magnetic and antimicrobial studies of transition metal complexes derived from hydrazone Schiff base ligand.” Journal of Saudi Chemical Society 2015, 19(1), 1–7.
- Hossain, Md E.; Sutradhar, M.; Paul, P.; Mitra, S. “Synthesis, characterization and antimicrobial activities of some mixed-ligand transition metal complexes.” Journal of Coordination Chemistry 2011, 64(17), 3078–3088.
- Chohan, Z. H.; Supuran, C. T. “Metal-based antibacterial and antifungal agents: Synthesis, characterization, and biological evaluation of sulfonamide-derived Schiff bases and their tin(II) complexes.” Journal of Enzyme Inhibition and Medicinal Chemistry 2008, 23(2), 240–247.
- Mohammad, F.; Ahmad, I. “Antifungal activity of some metal complexes of thiosemicarbazones derived from 4-acetylbiphenyl.” Transition Metal Chemistry 2007, 32(5), 661–666.
- Al-Shaalan, N. H. “Synthesis, characterization, and antimicrobial activity of Co(II), Ni(II), Cu(II) and Zn(II) complexes with new Schiff base derived from sulfamethoxazole.” Arabian Journal of Chemistry 2014, 7(6), 1071–1078.
- Mahmud, T.; Siddique, M.; Shahid, K.; Ali, S. “Antifungal and antibacterial evaluation of transition metal complexes of novel imine ligand.” Bioinorganic Chemistry and Applications 2017, 2017, Article ID 1549530.

