Aging is a natural process that every living thing goes through. The aging process is characterized by reduced body functions. This creates physical limitations, and it is not uncommon for people to depend on the help of others in carrying out their daily activities. Aging is often assumed to be a condition that is old and easily sick. Some of the factors that cause aging, for exampel: free radicals, apoptosis, decreased hormones, unhealthy lifestyle, environmental pollution, stress, lack of exercise and an unbalanced diet. Changes in lifestyle that occur in modern society today also accelerate the aging process. [1]
The aging process does not occur quickly, but gradually begins with reduced hormones. This hormone decline starts from the age of 25 years. At that time there were still no symtoms, but a decrease in hormone levels was detected through laboratory tests. This phase is the subclinical stage. In women who have entered the aging process at the clinical stage, it is marked by a significant decrease in several hormones, especially estrogen. Women who have experienced menopause have very low estrogen levels, which are less than 20 pg/ml [2]. This causes various complaints in cluding: hot flashes, vaginal dryness, decreased memory, depression, insomnia, anxiety, these symtomps will interfere with daily activities and ultimately affect quality of life [1].
The concept of Anti-Aging Medicine applies medical science and technology to detect, prevent and reverse aging-related conditions. Through the Anti- Aging Medicine approach, one way to deal with menopausal complaints is hormone replacement therapy [3]. Hormone replacement therapy is the therapy of choice to reduce complaints in women with menopausal complaints. However, the administration of hormone replacement therapy has limitations, namely the administration must be according to indications and during treatment there must be monitoring and evaluation. In long-term observation and in the loss of monitoring and evaluation during therapy, women who received this treatment showed an increased risk of breast, endometrial and ovarian cancer. For this reason, alternatives to estrogen must be sought to reduce complaints in menopausal women [4].
Basil (Ocimum basilicum L) is a plant that is widely used because it is easy to find and accessible to the public. Basil leaves contain lots of antioxidants such as flavonoids, carotenoids, riboflavin and thiamine. Antioxidants are useful for neutralizing the accumulation of free radicals and repair cell damage [5]. Apart from being antioxidants, flavonoids also have phytoestrogen activity. Phytoestrogens are chemical components that have estrogenic activity and can bind to estrogen receptors [6]. In many countries, basil is widely used as a medicinal plant. Basil leaves are used in traditional medicine to treat nausea, bloating, relieve complaints of flu, cough, headache and fever. In addition basil leaves are widely used as a complement to cooking. The fragrant aroma is popular and makes delicious dishes [7].
This study aims to prove that basil leaf extract can inhibited the decrease of ovarian follicles and increased estrogen levels in premenopausal female Wistar rats.
Methods
This research is an experimental post-test only control group design study using male rats, aged 10-18 months, with average body weight 200 grams. The material used in this research is the extract ethanol of basil leaves. The sample was divided into 2 groups, the treatment group which was given ethanol extract of basil leaf at a dosage of 0.50 g/kg BW and the control group which was given aquabidest every day orally for 21 days. After 21 days, blood was taken through the medial canthus of the orbital sinus to check estradiol levels using the ELISA method and the ovarian organs were taken for histological follicle counting. Number of ovarian follicle and estrogen levels were analyzed by Mann – Whitney non-parametric test.
Results
The results of the descriptive analysis of ovarian follicle in each group are shown in Table 1. The average of ovarian follicle in the control group was 5.39/LPB (95% CI 4.82 – 5.96) with a standard deviation was 1.145/LPB. The lowest of ovarian follicle in the control group was 4/LPB and the highest was 7/LPB. Furthermore, the average of ovarian follicle in the treatment group was 13.88/LPB (95% CI 12.41 – 15.36) with a standard deviation was 2.870. The lowest of ovarian follicle in the treatment group was 11/LPB and the highest was 19/LPB. The results of the Shapiro – Wilk test of ovarian follicle variable presented that the data in the control and treatment group were not normally distributed (P value <0.05).
Table 1. Descriptive analysis of ovarian follicle in the control and treatment groups
| Variables | Groups | n | Mean | SD | Minimal – Maximal | 95% CI | p-value |
| Number of Ovarian Follicle (/LPB) | Control | 18 | 5,39 | 1,14 | 4-7 | 4,82-5,96 | 0,001 |
| Treatment | 17 | 13,88 | 2,87 | 11-19 | 12,41-15,36 |

Figure 1. Comparison Graph of Ovarian Follicle Number at Control and Treatment Group
Figure 1 shows that number of ovarian follicle in the treatment group higher than the control group. The mean difference between the two groups was statistically significant (p < 0.001).
The results of the descriptive analysis of estrogen level in each group are shown in Table 2. The average of estrogen level in the control group was 207.02 ng/L (95% CI 203.47 – 210.56) with a standard deviation was 7.13 ng/L. The lowest of estrogen level in the control group was 191.52 ng/L and the highest was 214.5 ng/L. Furthermore, the average of estrogen level in the treatment group was 229.56 ng/L (95% CI 224.41 – 234.72) with a standard deviation was 10.02 ng/L. The lowest of estrogen level in the treatment group was 215.17 ng/L and the highest was 250.19 ng/L. The results of the Shapiro – Wilk test of estrogen level variable presented that the data in the control and treatment group were not normally distributed (P value <0.001).
Table 2. Descriptive analysis of estrogen level in the control and treatment groups
| Variables | Groups | n | Mean | SD | Minimal – Maximal | 95% CI | p-value |
| Estrogen Level (ng/L) | Control | 18 | 207,02 | 7,13 | 191,52-214,50 | 203.47 – 210.56 | 0,001 |
| Treatment | 17 | 229,56 | 10,02 | 215,17-250,19 | 224.41 – 234.72 |
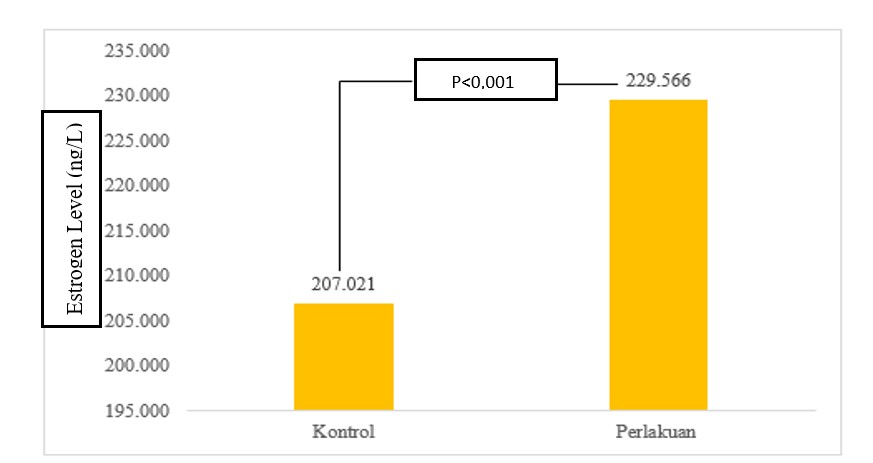
Figure 2. Comparison Graph of Estrogen Level at Control and Treatment Group
Discussion
The decrease in the number of follicles is caused by various processes, for example, the process of ovulation in each cycle per month and the presence of apoptosis. Apoptosis is the process of primordial follicles dying and stopping their growth [8]. In addition there are several other causes such as chemotherapy, radiation, gene mutations related to ovarian function, endometriosis cysts and ovarian cysts. Giving basil leaf ethanol extract helps prevent follicles from experiencing atretic. This is due to the content of flavonoids and antioxidants in basil leaves. This study proved that the number of follicles was higher in the treatment group after 21 days of administration of ethanol extract of basil leaves than the control group.
Basil leaves contain bioactive ingredients that can act as antioxidants, namely flavonoids, saponins, and tannins. besides that it also contains the vitamins ribofalvin, thiamine and ascorbic acid. The mechanism of flavonoids as antioxidants is direct scavenging of free radicals and chelating of transition metal elements, flavonoids can also act as an intracellular antioxidant through inhibition of free radical generating enzymes such as xanthine oxidase, lipoxygenase, protein kinase C, cyclooxygenase, microsomal monooxygenase, mitochondrial succinoxidase, and NADPH oxidase (9,5).
Saponin compounds work by increasing cellular defense mechanisms against oxidative stress [10]. Tannins contain hydroxyl groups which play an important role in producing antioxidant activity through chelating heavy metals, interacting with free radicals and ROS, inhibiting oxidative enzymes and regulating antioxidant enzymes[11].
Basil leaves also contain apigenin. Apigenin is a type of phytoestrogen that has strong antioxidant, anti-inflammatory and anti-cancer properties. In a study, it was found that in Poly Cystic Ovarian Syndrom cases, apigenin caused a significant increase in primary follicles, graaf follicles, and corpus luteum. In addition, there has also been a reduction in cystic follicles and atretic follicles [12].
The increase of estrogen levels is due to the content of phytoestrogens in basil leaves. Phytoestrogens are divided into 2 types, namely flavonoids and non-flavonoids.The mechanism of phytoestrogens in increasing estrogen is not clear, although many studies have proven it. Phytoestrogens have a phenolic ring so they can bind to estrogen receptors. To be able to carry out its function, estrogen needs to bind to its receptors. Phytoestrogens can work as estrogen receptor agonists or antagonists. This is influenced by many factors, including concentration, receptor type, presence or absence of endogenous estrogen, and target tissue. The affinity of phytoestrogens to bind estrogen receptor is weaker than estrogen [13]. When endogenous estrogen levels
decrease, there will be a lot of excess estrogen receptor that is not bound, so that even though the affinity is low, phytoestrogens can bind to these receptors [14].
Aryani in her research stated that giving foods containing phytoestrogens can increase estrogen levels in the blood. From the results of the report, it was stated that giving high-whey protein milk supplements containing phytoestrogens (0.092 mg/100 g) could increase estrogen levels. In addition, the mechanism of action of phytoestrogens in increasing endogenous estrogen is by increasing LH secretion by the anterior pituitary gland [15]. Rahayu found that administration of ethanol extract of basil leaves increased LH receptor expression and estradiol levels in premenopausal female Wistar rats. This is due to the interaction between LH and LHR which activates granulosa cells to produce estrogen [5].
Conclusion
Oral administration of 0.5 g/kg BW ethanol extract of basil leaves (Ocimum basilicum L) inhibited the decrease of ovarian follicles number and increase estrogen levels in premenopausal female Wistar rats.
References
- Pangkahila, W. 2019. Anti Aging Medicine : Tetap Muda dan Sehat. PT Kompas Media Nusantara.
- Stanczyk, F.Z., Clarke, N.J. 2014. Measurement of Estradiol—Challenges Ahead. The Journal of Clinical Endocrinology & Metabolism, 99(1): 56–58
- Pangkahila, W ., Wong, L.P. 2015. Evidence Base Anti Aging Medicine Hormone Replacement Therapy. TLC Publisher.
- Sugiritama, I. W., Adiputra, I. N. 2019. Potensi Antosianin dalam Manajemen Menopause.Jurnal Kesehatan Andalas, 8(1): 158.
- Rahayu, S., Widhaningrum, S. N., Saadah, M., Soewondo, A., Marhendra, A. P. W., Djati, M. S., Ciptadi, G. 2018. The effects of powder of Ocimum canum Sims. leaves on ovary LHR expression and serum estradiol levels in premenopausal rats (Rattus norvegicus). Fakultas Matematika dan Ilmu Pengetahuan Alam, Universitas Brawijaya
- Moreira, A.C., Silva, A.M., Santos, M.S., Sardão, V.A. 2014. Phytoestrogens as alternative hormone replacement therapy in menopause: What is real, what is unknown. J Steroid Biochem Mol Biol. 143:61-71.
- Bilal, A., Jahan N., Ahmed A., Naaz S., Habib S ., Hajra S. 2012. Phytochemical and Pharmacological Studies on Ocimum basilicum Linn-A Review, 4 (23), 73-83.
- Burger, H. G., Hale, G. E., Dennerstein, L., & Robertson, D. M. 2008. Cycle and hormone changes during perimenopause: The key role of ovarian function. Menopause, 15(4): 603–612
- Banjarnahor, S.D.S., Artanti, N. 2014. Antioxidant properties of flavonoids. Med J Indones 23 (4) : 239-244
- Kang, J. S., Kim, S. O., Kim, G. Y., Hwang, H. J., Kim, B. W., Chang, Y. C., Kim, W. J., Kim, C. M., Yoo, Y. H., & Choi, Y. H. 2016. An
exploration of the antioxidant effects of garlic saponins in mouse-derived C2C12 myoblasts. International Journal of Molecular Medicine, 37(1), 149–156.
- Tong, Z., He, W., Fan, X., & Guo, A. 2022. Biological Function of Plant Tannin and Its Application in Animal Health. Frontiers in Veterinary Science, 8(1) : 1–7.
- Darabi, P., Khazali, H., Natanzi, M.M. 2020. Therapeutic potentials of the natural plant flavonoid apigenin in polycystic ovary syndrome in rat model: via modulation of pro-inflammatory cytokines and antioxidant activity. Gynecological Endocrinology, 36(7): 582–587.
- Moreira, A.C., Silva, A.M., Santos, M.S., Sardão, V.A. 2014. Phytoestrogens as alternative hormone replacement therapy in menopause: What is real, what is unknown. J Steroid Biochem Mol Biol. 143:61-71
- Kim, S.H., Park, M.J. 2012. Effects of phytoestrogen on sexual development. Korean J Pediatr. 55(8):265-71
- Aryani, S. 2016. Pemberian susu suplemen tinggi protein whey (L-Men Platinum) dapat meningkatkan kadar testosteron dan estrogen pada tikus putih (Rattus norvegicus) jantan dengan aktivitas fisik sedang [Tesis]. Denpasar: Program Pascasarjana Magister Biomedik Universitas Udayana

