1.1. DIABETES:
Diabetes is a disorder where the blood glucose (sugar) level was higher than normal Blood glucose (sugar) level is known as Hyperglycemia.
Blood glucose levels are normally controlled by the hormone called insulin which is produced by beta cells of Langerhans in the pancreas (a gland which
lies just below the stomach)1
THIAZOLIDINONES-STRUCTURE
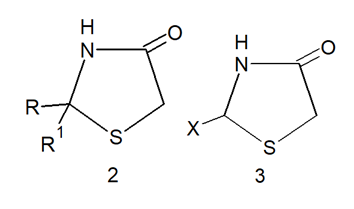
Thiazolidinones are derivatives of Thiazolidine with a carbonyl group at the 4-position.

Substituents in the 2-, 3-, and 5-positions may be varied, but the greatest difference in structure and properties is exerted by the group attached to the
carbon atom in the 2-position (R and R’ in 2 or X in3).2
Variations in the substituents attached to the nitrogen atom and the Methylene carbon atom are possible for the structures represented by 2 and 3.3
1.3 ANTIDIABETIC ACTIVITY OF THIAZOLIDINONE
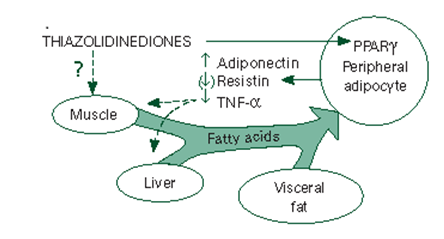
Figure No:1 Mechanism of action of thiazolidinediones
Thiazolidinones bind to the gamma form of the peroxisome proliferator-activated receptor (PPAR-) and stimulate the peripheral adipocytes to increase their uptake of free fatty acids which leads to the reduction of fats stored in the muscle, liver, and visceral fat deposits they also lead to an increase in the secretion of adiponectin and decrease in the production of resistin and tumor necrosis factor-α (TNF-α).amylase.4
1.4. THERAPEUTIC IMPORTANCE
The Thiazolidinone ring system represents a privileged structure in drug discovery. A large number of bioactive compounds containing this ring system are so vast that the complete range of their biological activities can be hardly classified. The nucleus contains the following therapeutic importance as shown in the figure.5
- INTRODUCTION TO SYNTHESIS OF TITLE COMPOUND:
Grindstone technique: The titled compounds are prepared by the grinding technique which is one of the green synthesis approaches. Green chemistry is the utilization of a set of principles that reduces the use of hazardous chemical substances in the design, manufacture, and application of chemical
products.6
Principle: The principle involved in the synthesis was a frictional force made between themortar and pestle.7
Application:
- Less hazardous chemical synthesis, with less energy and less time the compounds obtained.
- Solvent free synthesis.
- Cost effective
- Biocompatible.
- Biodegradable.
- Reliable and versatile.
- Convenient to handle.
- Simplicity.
- Safer and prevention of accidents.
- No use of higher temperature.
- Less wastage of chemicals (renewable).8
- Peroxisome proliferator-activated receptor γbinde
1. EXPERIMENTAL WORK
1.1. SYNTHETIC WORK
SCHEME OF WORK
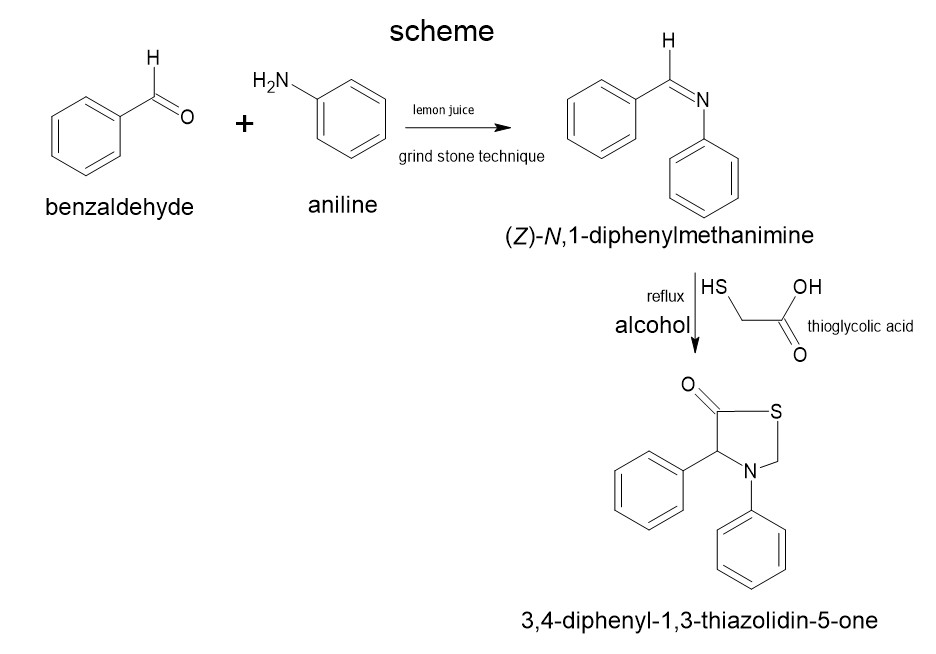
General method for the Synthesis of Title Compound :
The procedure for the synthesis of compounds consists of two steps .
Step 1 : Synthesis of schiff base:
0.01 mol aniline and 0.01 benzaldehyde is taken in a mortal .the reaction mixture was undergone continuous stirring at room temperature by using lemon juice as a natural catalyst for 2 hrs .while trituration water can be added .filtered and dried .to form the imines as a primary intermediate for the synthesis of thiazolidinones. This reaction involves the synthesis of Schiff base.
Step 2: Synthesis of thiazolidinone :
0.01mol of substituted Schiff base and 0.01mol of Thioglycolic acid and 1,2-dioxane wasadded and the reaction mixture was heated under reflux for 12
h. Concentrated, cooled and poured into the crushed ice. The solid thus separated was filtered, washed with water and recrystallized from ethanol.after the completion of reaction the reaction mixture was poured into crushed ice and the solid obtained was filtered, dried and recrystallised from ethanol.
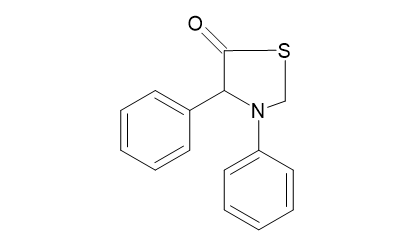
The compound that was synthesized is represented as below :
IUPAC name:3,4 -Diphenyl-1,3-Thiazolidin-5-One
PHYSICAL CHARACTERIZATION:
Solubility: Solubility of the synthesized title compound was tested using different solvents.
Melting point :the synthesizes compound has the melting point at 84 C.
ACTIVITY SCREENING
In vitro Antidiabetic activity:
Name of analysis method: glucose uptake assay
Procedure:
3T3-L1 adipocytes, were seeded at a density of ~1500 cells per well in a 96-well plate, differentiated and maintained for another 10 days prior to use. To assay glucose uptake, adipocytes were starved in 100 µl serum free adipocyte medium overnight (to enhance glucose uptake) then washed with PBS, followed by a incubation (40 min) in an glucose free medium (100 µl Krebs-Ringer-Phosphate-HEPES (KRPH) buffer with 2 % BSA) then stimulated either with insulin (PGZ) (10 μM), compounds (10 µg/ml) or PBS. 10 µl of 10mM 2-Deoxy glucose (DG) was added and the cells incubated for 20 min. The amount of glucose uptake was determined as per manufactures protocol using the Glucose uptake kit from Biovision (glucose uptake colorimetric assay kit, the 2-DG6P is oxidized to generate NADPH, which can be determined by an enzymatic recycling amplification reaction, color generated can be quantified colorimetrically at 412 nm.). The calculation was carried out keeping 100% glucose uptake for Pioglitazones (PGZ) was used as a standard drug. 9
2-DG uptake = Sa/Sv (pmol/µl or nmol/ml or µM)
Where: Sa is the amount of 2-DG6P (in pmol) in sample well calculated from Standard Curve. Sv is sample volume (in 20 µl) added into the sample well.
2. EXPERIMENTAL RESULTS
3.1 BIOLOGICAL ACTIVITY:
- In-vitro Antidiabetic Activity:
Antidiabetic activity of the synthesized derivatives was performed by the Glucose uptake assay and the results were tabulated below.
In-vitro Antidiabetic Activity:
Effect of compound PD-1) on 2-DGuptakein 3T3-L1 presence and absence of insulin:
Table no. 1
| S. No | Compound | OD(412) | 2DG6P(pmol) | 2-DGuptak(Pmol/µl) |
| 1 | Insulin(1microMol) | 3.04 | 174 | 11.5 |
| 2 | PD1 | 2.02 | 180 | 10.2 |
| 3 | PD1+Insulin | 10.7 | 460 | 23.4 |
| 10 | Pioglitazone(10Micro.Mol)+Insulin(1Micro.Mol) | 10.4 | 450 | 22.5 |
| 11 | Pioglitazone(10uuMicro.Mol) | 3.1 | 210 | 10.5 |
The results that are tabulated in Table clearly show that the synthesized thiazolidine shows the equipotent activity to that of insulin and pioglitazone as a standard. it also shows synergistic action when it is combined with insulin and pioglitazone.t There it has shown very good antidiabetic activity. By removing the molecules of pioglitazone which is the standard drug for diabetes mellitus, a novel compound thiazolidinone can be synthesized and even that shows the equipotent activity to that of pioglitazone.
3. DISCUSSION OF RESULTS
In this present research work, novel derivatives of Thiazolidinone containing pyridine derivatives were synthesized in a two-step facile procedure with good yields. The compound purification was done by the recrystallization process. Thiazolidinone derivatives were screened for In-vitro Anti-diabetic activity based on their In- silico anti-diabetic activity.
In– vitro Anti-diabetic activity:
The anti-diabetic activity of the title compounds was done by the Glucose uptake assay, the target is Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) which was performed by using Pioglitazone as the reference standard. Synthesized thiazolidinone shows the equipotent activity to that of insulin and pioglitazone as a standard. it also shows the synergistic action when it is combined with insulin and pioglitazone
PPARγ agonists that selectively modulate the receptor activity maintaining glucose homeostasis without the adverse effects associated with Thiazolidinone containing derivatives is a promising approach in diabetes research. In this context, the observed insulin-sensitizing effect through 2- DG uptake of compounds could be possibly attributable to mechanisms beyond PPARγ activation.
4. CONCLUSION
Novel thiazolidinone derivative are synthesized, characterized, and screened for in-vitro antidiabetic using respective standards.
The results of in-vitro antidiabetic screening revealed that the synthesized compound showed good activity when compared to standard. This may be due to the fact that the target.
The results of in-vitro antidiabetic screening revealed that the synthesized derivative have good antidiabetic activity when compared to that of currently marketed drugs pioglitazone and insulin.
Further research need to be carried out to know relationship between structure of thiazolidinone derivative and biological activity.
6. ACKNOWLDGEMENT:
The authors are thankful to the university for providing the funding to carry out this work.
7. REFERENCES
- Graham L. Patrick. An introduction to medicinal chemistry.1,94.
- Andres,C.J;Bronson,J.J.;D”Andrea,S.V.Deshpande,M.S.;Falk,P.J.;Grantyoung.Bioorg.Med.Chem.Lett.2000.10.715.
- Jerry R. et al., Experimental And Clinical Pharmacology, Australian Prescriber, 2004, 27 (3) ,67-70.
- M.G.Vigorita, A.Chimirri,S.Grasso,G.Fenech,J.Heterocycle.chem.1979, (16),257-261.
- Evans, R.M., Barish, G.D., Wang, Y.X.PPARs and the complex journey to Obesity. Nature Mediccine10, 355-361(2004).
- Y.R. Sharma. Elementary Organic Spectroscopy.1980
- Amin et al., synthesis and preliminary evaluation of some substituted coumarins as anticonvulsant agents. Bio org.Med.Chem.2008,16,5377- 5388.

