Introduction
Coordination polymers (CPs) are crystalline materials characterized by their highly ordered and extended architectures, composed of repeating coordination units that propagate in one, two, or three dimensions. These frameworks are constructed through the assembly of metal ions and organic ligands, which are connected via coordination bonds or, in some cases, through supramolecular interactions such as hydrogen bonding or π–π stacking interactions[^1,^2]. Among the various ligand systems employed, those containing both nitrogen atoms from pyridine rings and additional donor atoms like nitrogen or oxygen from acid hydrazide functionalities serve as versatile bifunctional coordinating agents. These ligands significantly enhance the potential for generating diverse CP structures and multimetallic complexes due to their ability to bind metal centers through multiple coordination sites[^3,^4].
The overall properties of coordination polymers—including their chemical reactivity, electronic behavior, and structural topology—are intricately governed by the specific nature of their metal-ligand interactions and spatial arrangement. These characteristics, in turn, directly influence their functional attributes such as electrical conductivity, luminescence, and magnetic responses[^5]. Moreover, CPs have attracted significant attention for their application potential in areas like heterogeneous catalysis, photocatalysis, and chemical sensing, owing to their tunable porosity, active metal centers, and high surface area[^6–^9].
Materials and Methods
Isonicotinoylhydrazide and formyl compounds were procured from Nice Chemicals and used without further purification. All organic solvents employed in the study were utilized as received.
Crystallographic data for the synthesized compound (DMN-INH) were collected using a CCD diffractometer equipped with monochromated MoKα radiation (λ = 0.71073 Å). Data acquisition and unit cell refinements were performed using the APEX2 software suite, while SAINT and XPREP were utilized for data reduction and preliminary structure analysis.
Structure solution and refinement were carried out using SIR92 and SHELXL, respectively. Molecular graphics and visual representations of the crystal structure were generated using ORTEP3.
Synthesis of the Metal Complex
A solution containing 10 mmol of isonicotinoylhydrazone dissolved in 15 mL of ethanol was prepared. To this solution, 10 mmol of zinc nitrate hexahydrate was added. The reaction mixture was then refluxed for 5 hours under constant stirring. Upon completion of the reaction, the resulting precipitate was filtered, washed, and dried under vacuum. The yield of the isolated complex was approximately 75%, with a melting point of 240°C.
Single crystals suitable for X-ray diffraction studies were obtained by slow crystallization of the complex from an acetic acid–methanol solvent mixture.
Results and Discussions
The reported zinc coordination polymer was found to be stable and insoluble in non-polar organic solvents, and was formulated by single-crystal X-ray analysis.
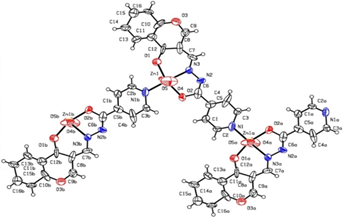
Figure 1: ORTEP diagram
Table 1.: Structural details and refinement parameter
| Empirical Formula | C16H10N3O5Zn |
| Formula Weight | 389.64 |
| Temperature | 293K |
| Wavelength | 0.71073Å |
| Crystal System | Monoclinic |
| Space Group | P21/n |
| Unit Cell Dimensions | a=9.3133(7) Å; alpha=90o |
| b=15.0916(11)Å; beta=97.810(3)o | |
| c=18.4344(15)Å; gamma=90o | |
| Volume | 2567.0(3) |
| Z, Calculated Density | 4, 1.008 |
| Absorption Coefficient | 0.977 |
| F(000) | 788 |
| Crystal size | 0.35×0.35×0.30 |
| Completeness to Theta=28.36 | 94.80% |
Table 2.: Important bond lengths [Å]
| Zn-O(1) | 1.998 | O(1)-C(12) | 1.35(4) |
| Zn-O(2) | 2.019(19) | N(2)-C(6) | 1.24 |
| Zn-N(1) | 2.04(2) | N(2)-N(3) | 1.41(2) |
| Zn-O(4) | 2.13(3) | N(3)-C(7) | 1.27 |
| Zn-O(5) | 2.150(17) | N(1)-C(2) | 1.25(3) |
| Zn-N(3) | 2.156(16) | N(1)-C(3) | 1.52 |
| O(3)-C(9) | 1.29 | N(1)-Zn | 2.04(2) |
| O(3)-C(10) | 1.41 | C(6)-O(2) | 1.36 |
Conclusion
2D zinc-coordination polymer was easily obtained by reacting isonicotinoylhydrazone and zincnitratehexahydrate under solvothermal method. Ethanol was used in the synthetic step. Good yield of isolated compound was obtained, and single-crystal x-ray diffraction revealed its polymeric nature.
Conflict of Interests
No conflicting financial interests exist for the authors.
References
- Janiak, C.; Engineering coordination polymers towards applications, DaltonTrans., 2003, 2781-2804.
- Dutta, B.; Dey, A.; Maity, S.; Sinha, C.; Partha Pratim Ray, P.P.; Mir, M.H. Supramolecular Assembly of a Zn(II)-Based 1D Coordination Polymer through Hydrogen Bonding and π···π Interactions: Crystal Structure and Device Applications, ACS Omega, 2018, 3, 12060-12067.
- Deng, W.T.; Liu, J.C.; Cao J. Syntheses, crystal structures and properties of four new coordination polymers involving a Schiff base ligand bearing an easily abstracted proton in the hydrazone backbone, Inorg. Chem. Commun., 35, 2013, 315-317.
- Afkhami, F.A.; Khandar, A.A.; Mahmoudi, G.; Amini, M.; Molins, E.; Garczarek, P.; Lipkowski, J.; White, J.M.; Alexander M. New cadmium(II) and zinc(II) coordination polymers derived from a pyridine hydrazone block: self-assembly generation, structural and topological features, and theoretical analysis, Inorganica Chimica Acta, 458, 2017, 68-76.
- Chen, C.T.; Suslickl K.S. Coord. Chem. Rev., 1993, 128, 293-322.
- Martins, N.M.R.; Mahmudov, K.T.; GuedesdaSilva, M.F.C.; Martins, L.M.D.R.S.; Guseinov, F.I.; Pombeiro, A.J.L. 1D Zn(II) coordination polymer of arylhydrazone of 5,5-dimethylcyclohexane-1,3-dione as a pre-catalyst for the Henry reaction, Catalysis Commun., 87, 2016, 49-52.
- Scaeteanu, G.V.; Maxim, C.; Badea, M.; Olar, R. Zinc(II) Carboxylate Coordination Polymers with Versatile Applications, Molecules, 2023, 28, 1132.
- Wu, Y.; Gu, Z.; Luo, W.; Wu, L.; Yulong Li, Y.; Xie, B.; Zou, L. Crystal structure, luminescent sensing and photocatalytic activity of a multifunctional hydrazone‑based zinc(II) coordination polymer, Trans. Met. Chem., 43, 2018, 673-681.
- Biswas, P.; Dastidar P. Anchoring Drugs to a Zinc(II) Coordination Polymer Network: Exploiting Structural Rationale toward the Design of Metallogels for Drug-Delivery Applications, Inorg. Chem., 2021, 60, 3218-3231.
