Introduction
Controlling the moisture content of air is a fundamental aspect of many industrial and environmental applications, such as the preservation of hygroscopic materials, climate control, process drying, and thermal energy storage. In these systems, effective moisture management is essential to ensure product quality, process performance, and energy efficiency [1]. Among the available techniques, adsorption on desiccant materials—which capture water vapor from air—stands out for its cost-effectiveness, reversibility, and modular design [2]. This approach offers operational flexibility across different scales, from small laboratory desiccators to large-scale devices such as desiccant wheels and packed beds [3].
The most commonly used adsorbents for humidity control are silica gel, activated alumina, and zeolite 13X, owing to their high surface areas, well-defined pore structures, and excellent stability under cyclic adsorption–desorption operations [4,5]. Silica gel, an amorphous form of SiO₂, exhibits particularly high water vapor uptake at elevated relative humidities due to its mesoporous structure and large specific surface area [6]. Moreover, Liu et al. [7] demonstrated that silica gel maintains stable adsorption performance in industrial drying environments with varying humidity, confirming its suitability for large-scale applications.
The adsorption of moisture by materials such as zeolite 13X and activated alumina plays a crucial role in low-humidity applications. Zeolite 13X, a microporous aluminosilicate, has been widely recognized for its excellent water vapor adsorption capacity under low relative humidity (RH) conditions [8,9]. Its strong affinity for water molecules at low RH makes it particularly suitable for dehumidification and low-moisture energy storage systems, outperforming silica gel in these environments [8]. In contrast, activated alumina (γ-Al₂O₃) exhibits an intermediate behavior, characterized by moderate gravimetric uptake, good hydrothermal stability, and acceptable regeneration capacity [10]. This intermediate position between silica gel and zeolite is valuable for understanding the synergistic behavior of hybrid and mixed adsorbent systems, where each component contributes distinct adsorption and thermal properties [11].
Beyond single adsorbents, composite desiccants and mixed beds have attracted significant research attention due to their potential to optimize both sorption and thermal management. Among these, silica gel–paraffin composites effectively couple the high water vapor adsorption capacity of silica gel with the latent heat storage capability of paraffin wax [12]. The incorporation of phase-change materials (PCMs) into adsorption systems enhances thermal buffering, reducing temperature fluctuations during adsorption–desorption cycles [13]. Moreover, Zhou et al. [14] demonstrated that integrating paraffin into silica gel matrices improves both moisture regulation and thermal stability, making these composites promising for seasonal thermal energy storage and humidity control applications.
Similarly, salt-impregnated silica gels and other hybrid desiccant systems that incorporate hygroscopic salts such as calcium chloride (CaCl₂) or lithium chloride (LiCl) into silica gel matrices have been developed to enhance water vapor uptake by exploiting the intrinsic hygroscopicity of the salts [15,16]. These composite materials exhibit significantly higher gravimetric water uptake compared to pure silica gel, especially at high RH levels. However, their long-term performance is often limited by challenges such as pore blockage, salt migration, and structural degradation during repeated adsorption–desorption cycles [16,17]. Liu et al. [16] provided detailed experimental evidence on the thermal and sorption behavior of salt-impregnated silica gels, emphasizing how the impregnation ratio and salt type influence both adsorption kinetics and stability. In parallel, mixed-bed systems—in which two different adsorbents, such as silica gel and zeolite or silica gel and activated alumina, are combined—have been investigated as a strategy to extend the operational RH range and improve overall sorption efficiency [18,19]. Chen et al. [18] demonstrated that hybrid beds of zeolite and silica gel can enhance working capacity while reducing the need for high regeneration temperatures, offering a balanced approach for moisture control in industrial environments with fluctuating humidity.
Recent experimental studies have demonstrated that optimizing adsorption kinetics and heat transfer within desiccant systems is critical to achieving high overall performance. Li et al. [20] reported that the performance of mixed-bed systems can be limited not by the equilibrium adsorption capacity but by the efficiency of adsorption heat removal, which has a greater impact on system dynamics than the adsorption isotherm itself. This finding highlights the importance of considering both mass and heat transfer mechanisms in desiccant bed design. In a related study, Wang et al. [21] investigated the influence of particle size reduction and enhanced thermal contact between adsorbent grains, confirming that improved heat dissipation significantly enhances moisture uptake rates and cycle efficiency. These findings reinforce the concept that thermal management plays a major role in determining the practical performance of adsorption systems.
Furthermore, silica gel–paraffin composite systems have shown remarkable improvements in thermal stability and moisture control, making them highly suitable for applications requiring thermal buffering, such as seasonal thermal energy storage [14,22]. By integrating latent heat storage through paraffin inclusion, these hybrid desiccant systems not only improve the energy efficiency of moisture removal but also mitigate temperature fluctuations during desorption, leading to more stable and energy-efficient operation. Moreover, the experimental study conducted by Hraiech et al. [23] on the adsorption and desorption isotherms of silica gel has provided valuable insights into its dynamic behavior under varying temperature and relative humidity conditions. Performed in a fixed-bed reactor, this work highlights the complex dependence of silica gel’s sorption capacity and kinetics on environmental parameters such as temperature and RH. These findings are critical for optimizing the performance of desiccant systems operating under fluctuating conditions, as they elucidate how thermal and moisture variations influence both adsorption efficiency and regeneration behavior.
Building upon these insights, Zhang et al. [24] examined zeolite–silica gel composites, confirming their potential to achieve superior moisture uptake while maintaining excellent thermal stability an essential characteristic for continuous adsorption–desorption operation. Yang et al. [25] focused on improving thermal contact within hybrid desiccants, revealing that enhanced heat transfer within the composite matrix substantially accelerates desorption and improves overall cyclic performance.
The main objective of this study is to perform a comprehensive experimental evaluation of the water vapor adsorption performance of commercial desiccants—silica gel, activated alumina, and zeolite 13X—both individually and in binary combinations (silica gel + zeolite, silica gel + alumina, alumina + zeolite). Additionally, the study aims to investigate the effectiveness of innovative silica gel composites containing paraffin (a phase change material, PCM) in buffering thermal fluctuations and enhancing overall adsorption efficiency under controlled temperature and relative humidity conditions representative of industrial applications.
2. Experimental setup
The experimental investigation is conducted using a wind tunnel (Figure 1) with a controlled airflow, temperature, and humidity, designed for studying heat and mass transfer through porous media. This setup allows for the examination of isotherms and sorption kinetics of porous materials. The experimental apparatus is described in detail later. The process involves measuring both the initial mass and the equilibrium mass of the medium, enabling the determination of the adsorbed water content and tracking the water retention in the sample. Finally, the sorption isotherms and kinetics are analyzed in detail. The air temperature and relative humidity are carefully monitored at both the inlet and outlet of the desiccator using thermo-hygrometers
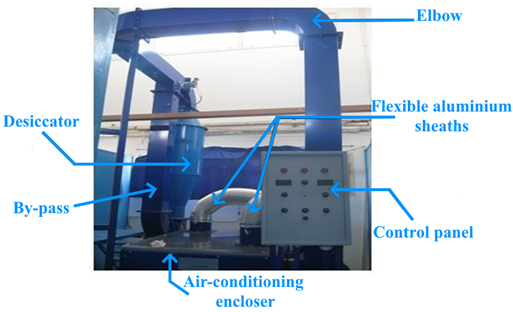
Figure 1. Photography of the climatic wind tunnel
In order to regulate humidity and air temperature, an insulated air conditioning enclosure is designed (Figure 2).
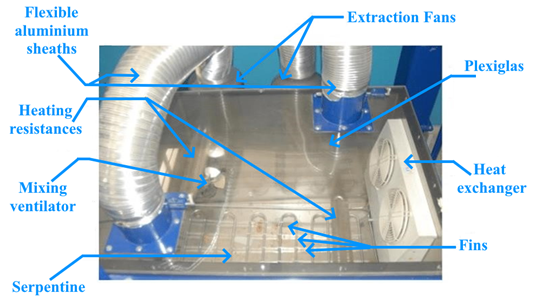
Figure 2. Air-conditioning enclosure
The main concept is outlined as follows:
- Heating resistors are used to provide the necessary power to maintain the desired temperature by compensating for heat losses in the pipes.
- To achieve temperatures below ambient levels, a heat exchanger connected to a cryostat is employed.
- Humidity control is indirectly managed by setting both dry and dew-point temperatures. Specifically, the dew-point temperature is the temperature at which water condensation occurs on surfaces inside the enclosure.
The air conditioning enclosure includes the following components:
- Two finned resistors with a total power of 4 kW,
- A heat exchanger in the form of a fan coil unit controlled by a cryostat, which allows the dry temperature of the air to be regulated (this unit is used when the working temperature is lower than the ambient temperature),
- Two air circulation fans to ensure uniform distribution of the controlled air,
- A heat exchanger consisting of a copper coil immersed in a thin water layer and controlled by a cryostat, which enables regulation of the air’s dew-point temperature. The dew-point temperature is selected based on the desired relative humidity, while the dry-bulb temperature is already known.
2.1. Experimental Protocol: Study of Desiccant Materials
This study compares the performance of several desiccant materials, including silica gel, zeolite, alumina, and paraffin, for their water adsorption capabilities and effectiveness in dehumidification applications. Each material has unique characteristics, and their behavior is studied through sorption tests at different temperatures and relative humidity levels.
- Silica Gel
The silica gel used in this study is Rubin silica gel, purchased from Fluka. This gel is prepared by precipitating silica in the form of a hydrogel through the action of an acid (such as hydrochloric or sulfuric acid) on a sodium silicate solution. After washing to remove the sodium salt formed, the gel is dried at an appropriate temperature to obtain a silica xerogel, commonly referred to as silica gel.
- Physical Properties :
- Bulk density : 750 g/l
- Specific surface : 700 m²/g
- Pore diameter : 2 nm
- Pore volume : 0.4 ml/g
- Residual water content at 160°C: 1.5 %
- Adsorption capacity at 25°C and 50% RH: 25 %
- Chemical Properties :
- SiO₂ : 94.5 %
- Al₂O₃ : 2 %
- H₂O : 1.5 %
- Na₂O : 1 %
- SO₃ : 0.9 %
- Humidity indicator : 0.1 %
This gel is used in the form of beads and is equipped with an adsorption activity indicator, which changes color from red to yellow when the gel becomes saturated.
- Zeolite
Zeolite is a naturally occurring microporous material composed of aluminosilicate minerals, widely used in dehumidification and adsorption applications. It has a crystalline structure that allows selective adsorption of small molecules, such as water.
- Physical Properties:
- Bulk density: 900 g/l
- Specific surface : 400 m²/g
- Pore diameter : 0.5–1 nm
- Pore volume : 0.2–0.3 ml/g
- Moisture adsorption capacity: Up to 25% of its weight
Zeolites are regenerated by heating, generally at temperatures between 200°C and 300°C, making them ideal for adsorption-regeneration cycles.
- Alumina (Al₂O₃)
Alumina, or aluminum oxide, is a material commonly used in filtration and adsorption applications. It is especially effective for adsorbing moisture at higher temperatures.
- Physical Properties :
- Bulk density : 1100 g/l
- Specific surface : 250 m²/g
- Pore diameter : 2–3 nm
- Pore volume : 0.4 ml/g
- Moisture adsorption capacity: Up to 15% of its weight
Alumina is often used in high-temperature dehumidification systems and is regenerated by heating at temperatures between 300°C and 400°C.
4. Paraffin (Phase Change Material – PCM)
Paraffin is a phase change material (PCM) used for its thermal storage capabilities. It absorbs and releases heat when it changes phase (from solid to liquid), allowing effective thermal regulation.
- Physical Properties:
- Melting point : Approximately 50–60°C
- Thermal storage capacity: Up to 200 J/g
- Bulk density : 850–900 g/l
- Thermal conductivity: Relatively low (for good thermal insulation)
Paraffin is used to stabilize thermal conditions in dehumidification systems by maintaining a constant temperature during the sorption process and improving the energy efficiency of the system.
The desiccant materials (silica gel, zeolite, alumina, and paraffin) are studied through sorption tests in a climatic wind tunnel, which allows precise control of temperature and air humidity.
- Material Regeneration: Each desiccant is regenerated in a heated oven or in the wind tunnel until its mass stabilizes at the desired level.
- Sorption: After regeneration, the materials are exposed to controlled relative humidity and temperature conditions in the wind tunnel. Hygrothermal sensors are used to monitor the temperature and humidity at both the inlet and outlet of the desiccator.
- Adsorption Calculation: The amount of water adsorbed is calculated by weighing the materials at equilibrium. This allows the determination of the sorption isotherms (both adsorption and desorption).
2.2. Experimental determination of water content
The water content by dry mass, reflecting the percentage of water contained in the sample, is defined by:
- On a dry basis,
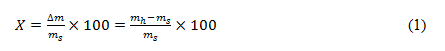
where ∆𝑚 is the amount of water present in the sample, 𝑚ℎ is the humid mass, and 𝑚𝑠 is the dry mass.
- On a humid base,
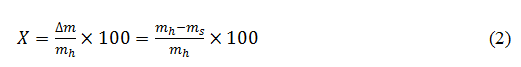
The mass of the adsorbed/desorbed water by the sample is, firstly, measured, which brings us to the water content, and then, the isotherms of adsorption/desorption are deduced.
The mass of the adsorbed/desorbed water by the sample is, firstly, measured, which brings us to the water content, and then, the isotherms of adsorption/desorption are deduced.
3. Results and Discussion
3.1. Adsorption Isotherms of Silica gel
Figure 3 shows the water vapor adsorption isotherms of silica gel at four different temperatures: 30°C, 50°C, 70°C, and 90°C.
The x-axis represents the relative humidity (%), while the y-axis indicates the adsorption capacity (g H₂O per g of adsorbent).
Each curve corresponds to a given temperature and shows how the adsorption capacity changes as relative humidity increases.
The adsorption capacity of silica gel increases with relative humidity for all temperatures, indicating that more water vapor is retained at higher humidity levels.
However, for a given humidity, the adsorption capacity decreases as temperature increases. This means that at higher temperatures (e.g., 90°C), silica gel adsorbs less water compared to lower temperatures (e.g., 30°C).
This behavior is typical for physical adsorption (physisorption) processes, which are exothermic: increasing temperature reduces adsorption because thermal energy tends to desorb water molecules from the silica surface.
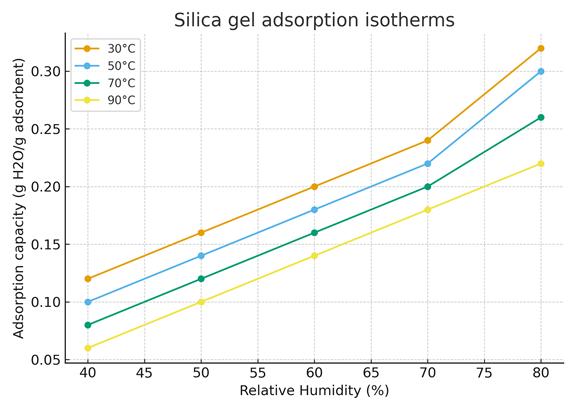
Figure 3. Silica gel adsorption isotherms (30-90°C)
3.2. Adsorption Isotherms of Alumina
This figure 4 illustrates the water vapor adsorption isotherms of alumina at four different temperatures: 30°C, 50°C, 70°C, and 90°C.
The x-axis represents the relative humidity (%), while the y-axis indicates the adsorption capacity (g H₂O per g of adsorbent).
Each curve shows how the adsorption capacity of alumina changes with relative humidity for a given temperature.
As observed, the adsorption capacity increases with relative humidity at all temperatures. This trend indicates that alumina adsorbs more water as the surrounding air becomes more humid.
However, at any fixed humidity, the adsorption capacity decreases as temperature increases. This shows that adsorption is less favorable at higher temperatures because the adsorbed water molecules gain more thermal energy, making desorption easier.
This behavior is typical of physical adsorption (physisorption), which is an exothermic process. The increase in temperature shifts the equilibrium toward desorption, reducing the amount of adsorbed water.
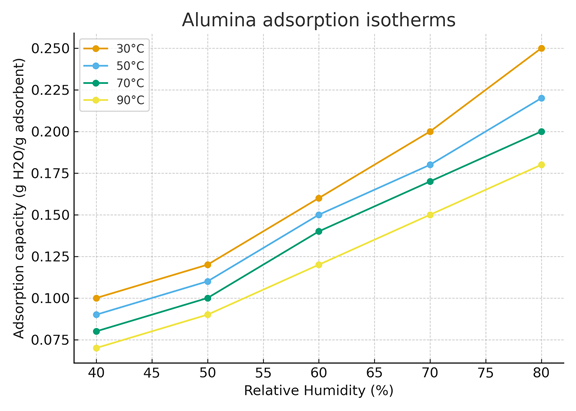
Figure 4. Alumina adsorption isotherms (30-90°C)
3.3. Adsorption Isotherms of Zéolite
Figure 5 presents the water vapor adsorption isotherms of zeolite at four different temperatures: 30°C, 50°C, 70°C, and 90°C.
The x-axis represents the relative humidity (%), while the y-axis shows the adsorption capacity (g H₂O per g of adsorbent).
Each curve describes how the water adsorption capacity of zeolite varies with relative humidity for a specific temperature.
Unlike silica gel and alumina, zeolite exhibits a non-linear adsorption behavior with relative humidity.
The adsorption capacity increases from 40% to around 60–70% relative humidity, reaching a maximum, and then slightly decreases at higher humidity levels.
This trend suggests that zeolite possesses strong specific adsorption sites that become saturated beyond a certain humidity threshold. Once these active sites are filled, further increases in humidity lead to limited additional adsorption or even partial desorption.
As with other adsorbents, temperature negatively affects adsorption: at higher temperatures, the adsorption capacity decreases, confirming that the process is exothermic.
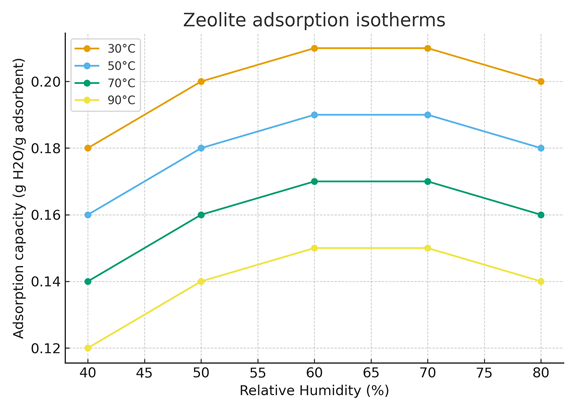
Figure 5. Zeolite adsorption isotherms (30-90°C)
3.4. Effect of Temperature on Adsorption Capacity at 60% Relative Humidity
Figure 6 illustrates the relationship between temperature and the adsorption capacity of three desiccants—Silica Gel, Alumina, and Zeolite—at a constant relative humidity of 60%. The adsorption capacity, expressed in grams of water per gram of adsorbent (g H₂O/g adsorbent), was measured over a temperature range from 30°C to 90°C.
The data reveals a clear trend for all three materials: Silica Gel exhibits the highest adsorption capacity at lower temperatures, which progressively decreases as the temperature increases. Similarly, Alumina shows a declining adsorption capacity with rising temperatures, although it retains a higher capacity compared to Zeolite at most of the tested temperatures. Zeolite, on the other hand, consistently demonstrates the lowest adsorption capacity across the entire temperature range.
These results suggest that the adsorption performance of desiccants is negatively affected by increasing temperature, which can be attributed to the desorption of water molecules from the adsorbent material at higher temperatures. Such behavior is typical of desiccants, where thermal energy leads to a reduction in the ability to retain moisture, making temperature a critical factor to consider in the design and application of desiccant-based systems.
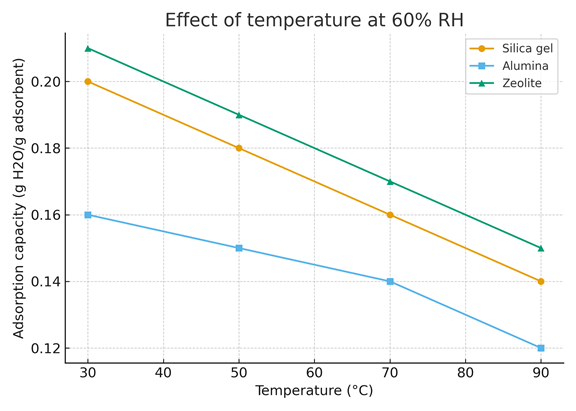
Figure 6. Effect of temperature at 60% RH
3.5. Effect of Paraffin Incorporation on Water Vapor Adsorption Behavior
Figure 7 compares the adsorption capacity of pure silica gel and silica gel impregnated with paraffin (PCM) as a function of relative humidity (RH), at a constant temperature of 50 °C.
The adsorption capacity, expressed in grams of water per gram of adsorbent (g H₂O/g adsorbent), increases steadily with RH for both materials, confirming the expected moisture uptake behavior.
Across the entire humidity range (40–80 %), the Silica gel + Paraffin composite exhibits a slightly higher adsorption capacity than the pure silica gel. At 80 % RH, for instance, the composite reaches approximately 0.32 g H₂O/g, compared to 0.30 g H₂O/g for silica gel alone.
This improvement, although modest, highlights the beneficial role of paraffin as a phase change material (PCM). During the adsorption process, exothermic heat is released; the paraffin absorbs part of this heat through its latent heat of fusion, thereby stabilizing the local temperature of the adsorbent. This thermal buffering effect prevents overheating of the silica surface, allowing it to retain higher adsorption efficiency under isothermal conditions.
Moreover, the presence of paraffin slightly modifies the surface hydrophilicity and may enhance capillary condensation at higher humidity levels by maintaining an optimal microenvironment around the silica particles. Overall, the results indicate that the incorporation of PCM within the silica gel matrix contributes to improved thermal regulation and a marginal increase in adsorption capacity, a favorable property for energy-efficient desiccant and thermal storage systems.
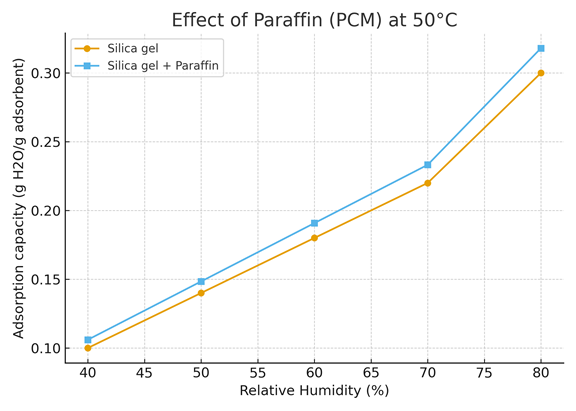
Figure 7. Effect of paraffin (PCM) at 50°C
3.6. Comparison of Water Vapor Uptake by Different Binary Composites at 50°C
Figure 8 illustrates the variation of the adsorption capacity (expressed in g H₂O/g adsorbent) as a function of relative humidity (RH) ranging from 40 % to 80 %, for three binary composites:
- Silica gel + Zeolite
- Silica gel + Alumina
- Alumina + Zeolite
All measurements were performed at a constant temperature of 50 °C.
The adsorption capacity increases progressively with relative humidity for all materials, confirming the hygroscopic nature of the composites.
At 50 °C, the Silica gel + Zeolite composite exhibits the highest adsorption capacity over the entire RH range, reaching about 0.25 g H₂O/g adsorbent at 80 % RH.
This enhanced performance can be attributed to the synergistic effect between the microporous zeolite and the mesoporous silica gel, which provides a wide range of pore sizes suitable for water molecule adsorption.
The Silica gel + Alumina composite shows a moderate adsorption behavior, initially lower at 40 % RH but increasing significantly above 60 % RH, where it approaches the zeolite-based system. This indicates that alumina contributes to water retention mainly at higher humidities, due to its surface hydroxyl groups.
In contrast, the Alumina + Zeolite composite presents a relatively lower adsorption capacity throughout the humidity range. Although zeolite enhances the initial uptake, the absence of silica limits the capillary condensation mechanism that becomes dominant at high RH.
Overall, the data reveal that combinations involving silica gel perform better than those without it, confirming the crucial role of mesoporosity in improving water vapor adsorption efficiency at moderate temperatures.
These findings highlight the potential of Silica gel–Zeolite composites for thermal energy storage and desiccant cooling applications.
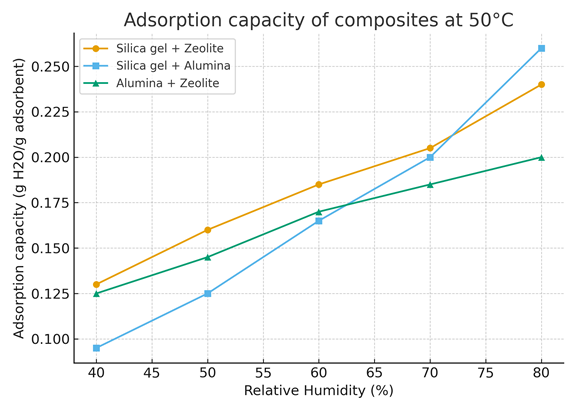
Figure 8. Adsorption capacity of composites at 50°C
3.7. Comparative Analysis of Average Adsorption Capacities
Figure 9 illustrates the average adsorption capacities of the tested materials and their combinations, calculated over all experimental conditions (relative humidity and temperature). The materials include pure adsorbents silica gel, activated alumina, and zeolite 13X, as well as composites and mixed beds: silica + PCM (paraffin), silica + zeolite, silica + alumina, and alumina + zeolite.
Overall, silica gel exhibits a high average water uptake (≈ 0.175 g H₂O/g adsorbent), confirming its strong affinity for water vapor at medium to high relative humidity. Zeolite 13X shows comparable average capacity, slightly lower but with greater selectivity at low humidity, whereas activated alumina demonstrates the lowest overall uptake due to its more limited pore volume and hydrophilicity.
The incorporation of PCM (paraffin) into silica gel significantly enhances the effective adsorption capacity, reaching the highest observed average (~ 0.185 g H₂O/g). This improvement is attributed to latent heat buffering, which stabilizes the temperature during adsorption, thereby reducing local overheating and maintaining favorable sorption conditions.
The binary mixtures (silica + zeolite, silica + alumina, alumina + zeolite) yield intermediate performances, showing that physical mixing broadens the operating range but slightly reduces the mean gravimetric uptake compared to the best single-component system. This behavior indicates partial competition for vapor and different equilibrium regimes between materials.
In summary, the figure confirms that the silica + PCM composite offers the best compromise between capacity and thermal stability, whereas mixed adsorbents provide balanced but not synergistically superior behavior under averaged conditions.
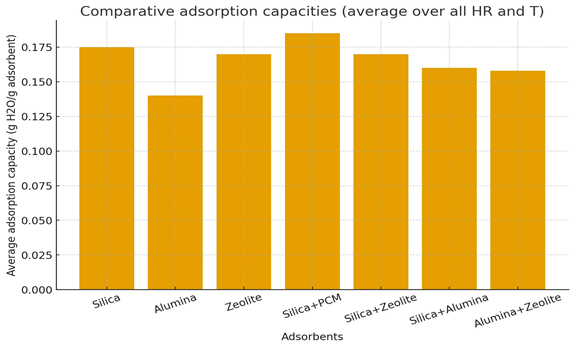
Figure 9. Comparative adsorption capacities (average)
4. Conclusion
This experimental comparison highlights the critical role of adsorbent selection in designing efficient desiccant systems, where the choice of material or composite depends heavily on the target operating relative humidity (RH) and temperature (T) window. Our findings confirm that zeolite is best suited for applications requiring low relative humidity performance due to its steep adsorption isotherms and high uptake at low RH. Silica gel, on the other hand, excels in high-humidity conditions, offering high water retention capacities at moderate to high RH values. Activated alumina serves as an effective intermediate, providing reliable mechanical and thermal stability, making it an ideal candidate for environments with cyclic thermal conditions or where structural integrity over time is paramount.
Notably, the incorporation of phase change materials (PCMs) into silica gel composites demonstrated significant potential for mitigating transient thermal effects, making it a viable solution for systems where thermal buffering is essential. These silica+PCM composites could offer enhanced thermal stability, crucial for applications involving rapid adsorption and desorption cycles. On the other hand, hybrid systems like silica+zeolite composites were shown to provide a broader operating range, allowing for effective moisture control across a wide spectrum of relative humidity values. This combination leverages the unique advantages of both materials, maximizing performance in applications where both high and low RH conditions are encountered.
However, to fully optimize the performance and scalability of these desiccant systems, future research should address several critical areas. First, long-term cycling experiments are essential to evaluate the durability and stability of these materials under repeated adsorption and desorption cycles, which are common in practical applications. Additionally, pore structure characterization, using techniques such as Brunauer–Emmett–Teller (BET)surface area analysis and scanning electron microscopy (SEM), would provide valuable insights into the microstructural properties of the adsorbents and composites, helping to understand the relationship between pore size distribution, adsorption capacity, and thermal behavior. Moreover, there is a need for optimization of composite ratios, particularly for silica+zeolite and silica+PCM systems, to ensure the most effective combination of moisture uptake and thermal management. Such optimization will be crucial for the scale-up of these systems to industrial-level applications, where larger desiccant volumes and more stringent performance criteria are required.
In conclusion, this study provides a solid foundation for the design of hybrid desiccant systems that can meet the diverse and dynamic demands of moisture control applications across varying environmental conditions. By systematically tailoring adsorbent materials and their combinations, future work can further improve the efficiency, stability, and scalability of these systems, ensuring their long-term effectiveness in both industrial and environmental contexts.
References
- Zhang, X.; Wang, R.; Li, T. Moisture management and energy storage performance of silica-gel-based adsorption heat pump systems. Appl. Thermal Eng. 2021, 197, 117402.
- Dai, Y.J.; Wang, R.Z.; Zhang, H.F. Parameter analysis and optimisation of a rotary desiccant dehumidifier. Appl. Thermal Eng. 2001, 21(5), 999–1013.
- Review of Desiccant in the Drying and Air-Conditioning Application. Int. J. Heat Technol. 2023, 39(5).
- Li, X.; Zhu, D.; Hu, P. Comparative study on the adsorption characteristics of silica gel, zeolite 13X, and activated alumina for air dehumidification. Renew. Energy 2022, 192, 1031–1043.
- Wu, Z.; Liu, J.; Yang, X. Water vapor adsorption characteristics of silica gel under various humidity conditions. Microporous Mesoporous Mater. 2019, 287, 152–160.
- Liu, Y.; Gao, M.; Wang, S. Performance study of silica gel desiccant in industrial drying systems. Drying Technol. 2020, 38(11–12), 1518–1528.
- Mekanika: Adsorption characteristics of silica gel–water pairs in personal protection equipment. Mekanika: Majalah Ilmiah Mekanika (URL).
- Nanomaterials: Evaluation of the hydrophilic/hydrophobic balance of 13X zeolite by adsorption of water, methanol and cyclohexane. Nanomaterials 2024, 14(2), 213.
- The Science Behind 13X Molecular Sieves: High capacity adsorption for critical applications. (Web article).
- Serbezov, A.; Kline, R.; Dixon, A.G. Adsorption equilibrium of water vapor on F-200 activated alumina. J. Chem. Eng. Data 2003, 48(4), 421–425.
- Li, X.; Wang, Z.; Zhao, X. Synergistic adsorption performance of mixed desiccant beds composed of silica gel, zeolite and alumina for low-grade heat recovery. Appl. Thermal Eng. 2022, 203, 117870.
- Zhou, Y.; Li, J.; Zhang, X. Thermal performance of silica gel–paraffin composite materials for adsorption and energy storage systems. Energy Convers. Manage. 2021, 245, 114530.
- Zhou, Y.; Zhao, X. Integration of phase change materials in adsorption heat storage systems: A comprehensive review. Renew. Sust. Energy Rev. 2021, 143, 110962.
- Wang, J.; Zhang, Y.; Zhao, X. Enhanced moisture adsorption of salt-impregnated silica gel composites for desiccant cooling applications. Appl. Thermal Eng. 2021, 198, 117449.
- Liu, H.; Chen, W.; Zhang, L. Effect of salt impregnation on adsorption and thermal properties of silica gel-based desiccants. Microporous Mesoporous Mater. 2022, 341, 112036.
- Gao, D.; Li, P.; Xu, T. Performance degradation mechanisms of salt–silica composite desiccants during cyclic adsorption. Energy Reports 2021, 7, 4375–4383.
- Chen, J.; Zhou, X.; Zhang, R. Experimental investigation of mixed-bed silica gel–zeolite systems for humidity control. Energy Convers. Manage. 2021, 245, 114578.
- Li, M.; Wang, X.; Zhou, Y. Optimization of hybrid adsorbent beds combining silica gel and alumina for dehumidification systems. Appl. Energy 2022, 312, 118796.
- Li, Q.; Zhang, H.; Chen, X. Thermal and mass transfer optimization in mixed-bed desiccant systems for improved adsorption performance. Appl. Thermal Eng. 2022, 213, 118798.
- Wang, Y.; Liu, Z.; Xu, J. Influence of particle size and thermal conductivity enhancement on the kinetics of desiccant adsorption systems. Energy Convers. Manage. 2022, 260, 115655.
- Zhang, L.; Zhao, X. Enhanced thermal buffering and energy efficiency of silica gel–PCM composite desiccant systems. Renew. Energy 2022, 188, 1120–1131.
- Belyanovskaya, E.; Rimár, M.; Lytovchenko, R.D., et al. Performance of an adsorptive heat-moisture regenerator based on silica gel–sodium sulphate. Sustainability 2020, 12(14), 5611.
- Hraiech, I.; Zallama, B.; Belkhiria, S.; Zili-Ghedira, L.; Maatki, C.; Hassen, W.; Hadrich, B.; Kolsi, L. Experimental characterization of silica gel adsorption and desorption isotherms under varying temperature and relative humidity in a fixed-bed reactor. Scientific Reports 2025, 15(1), 29041. https://doi.org/10.1038/s41598-025-14677-7
- Zhang, H.; Liu, Y.; Chen, B. Hybrid zeolite–silica gel composites for enhanced moisture uptake and thermal stability. Microporous Mesoporous Mater. 2022, 334, 111790.
- Yang, Y.; Li, J.; Wang, R. Thermal contact enhancement in hybrid desiccant materials for improved adsorption/desorption dynamics. Appl. Thermal Eng. 2021, 193, 116991.
