Introduction:
In the quest for a sustainable protocol for chemical synthesis, there’s a growing demand to replace traditional industrial processes with more eco-friendly, cost-effective, and energy-effective heterogeneous catalytic processes. Depending on the reaction type, heterogeneous catalysis is advantageous over homogeneous catalysis. When considered heterogeneous catalysis, non-noble metal based solid base catalysts including alkali and alkaline earth oxides, pure alkali metals or their compounds, basic zeolites, immobilized organic bases, hydroxyapatites, and clays such as hydrotalcite are special type of heterogeneous catalysts and gaining prominence in certain transformations due to many important reasons.
The solid-base catalysts provide economic feasibility and exceptional stability, making them appealing for diverse industrial applications. It is well-known that non-noble metal containing solid-base catalysts are typically made from more common and less expensive precursors. These catalysts are also found to enhance the selectivity and efficiency in many chemical reactions. These solid-base catalysts encompass Brønsted and Lewis basic activity centers, offering electrons or accepting protons from reactants. They can operate by adsorbing and donating hydroxide ions (OH-) or accepting and donating electrons, making them essential for acid-base reactions in various catalytic processes.
Figures 1 and 2 disclose the types of common non-noble metal based solid-base catalysts including hydrotalcite, metal oxides, metallic salts, supported base catalysts, and zeolites, and their application in a wide variety of organic syntheses.1 Although non-noble metal-based catalysts have relatively lower activity than the noble-metal catalysts, additionally, their readily accessible, acceptable lifetime, and good regeneration ability are unique remarks of such materials.2
Alkaline earth metal oxides: Alkaline earth metal oxide catalysts are a type of solid-base heterogeneous catalysts that are composed of oxides of alkaline earth metals. Alkaline earth metals are a group of elements in the periodic table that includes beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). Alkaline earth metal oxide catalysts are known for their basic properties and are particularly effective in base-catalyzed reactions. They are often used in the conversion of organic compounds and play a crucial role in the chemical and petrochemical industries, as well as in environmental applications like carbon capture and storage.3
The fundamental nature of these catalysts relies on the interplay between a surface metal ion acting as a Lewis acid and an oxygen ion exhibiting Brønsted basic characteristics. These catalysts are cost-effective, readily available, reusable, and corrosion-resistant. However, their effectiveness can decline due to the poisoning of basic sites through the strong adsorption of free fatty acids (FFA) and water on the surface. This results in a reduction in activity with each subsequent use due to side reactions involving saponification. Various studies have established a correlation between the basic strength of alkaline earth oxides and their catalytic activity, with the order being BaO > SrO > CaO > MgO.4
MgO and CaO type single/mono oxides have found extensive application as heterogeneous catalysts in the field of catalysis, owing to their distinct attributes. Their pronounced basicity, generous surface area, thermal resilience, and regenerative capacity endow them with substantial worth as catalysts in numerous industrial and laboratory processes where a robust base is essential. This includes C-C and C-heteroatom bond forming reactions like transesterification, aldol condensation, Knoevenagel condensation, acetylation, coupling reactions, oligomerization, isomerization, and so forth. Furthermore, their non-corrosive nature and eco-friendly qualities contribute to their widespread acceptance across diverse applications. However, it is considerable that at higher temperatures the particles of single metal oxides can agglomerate and form larger and less active particles. Thus, the catalytic activity of single metal oxides may be reduced possibly. However, the synergistic interactions in binary metal oxides refer to the cooperative or enhanced catalytic behavior that arises when two different metal oxides are combined to form a binary oxide catalyst.5 These interactions can lead to improved catalytic activity and selectivity compared to using single/individual alkaline earth metal oxides. Hence, from the above-mentioned statements we are interested in presenting the catalytic nature of binary alkaline earth oxide mixture especially MgO-CaO in various reactions. This review gives a summary of the emergence of CMBOs i.e., CaO-MgO based binary oxides as efficient heterogeneous solid-base catalysts for a variety of reactions in synthetic organic/ inorganic chemistry as well as useful materials in sorption studies.
The main contents of this micro review are divided into the following three (3) sections:
- CMBO catalyzed transesterification reactions
- CMBO catalyzed C-C, C-heteroatom bond forming organic synthesis other than transesterification
- CMBO assisted Sorption studies
- CMBO catalyzed transesterification reactions
Transesterification is an important industrial chemical process that allows the modification of ester compounds, including the production of valuable products like biodiesel, which is a renewable and environmentally friendly alternative to fossil fuels. The reaction typically involves triglycerides (Vegetable oils are mainly esters of fatty acids and glycerol) and an alcohol, such as methanol or ethanol, in the presence of a catalyst, and produces fatty acid methyl esters i.e., as biodiesel. Generally, sodium hydroxide (NaOH) and potassium hydroxide (KOH) are conventional, cost-effective homogeneous catalysts employed widely in industrial transesterification processes. These catalysts are reported to promote the reaction efficiently at low temperatures and pressure for yielding good conversion rates and large-scale production without the need for intermediate steps. Nevertheless, it’s important to consider that these catalysts are highly hygroscopic, readily absorbing moisture from the surrounding environment during storage, which can impact their performance by generating water when mixed with alcohol reactants, thereby influencing overall yields. Further, the removal of homogeneous catalysts is not easy or not at all possible and points out the necessity of exploration of heterogeneous catalysts to reduce biodiesel production costs. Various heterogeneous catalysts showed significant potential in converting vegetable oils into biodiesel. Solid heterogeneous catalysts, being non-corrosive and suitable for use in fixed-bed reactors, offer cost-effective and environmentally friendly processes (Scheme 1). Furthermore, their recyclability and compatibility with continuous mass production make them an attractive choice for biodiesel manufacturing. In this context, the efficacy of CaO-MgO binary metal oxide catalysts is highly considered to meet the requirements of sustainable transesterification both in small- and large-scale processes.
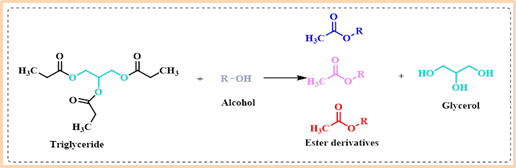
Scheme 1: Transesterification reaction and products
CMBO catalysts were extensively used in the transesterification process by different compositions, precursors, and synthetic methods (Figur 3). Ibrahim et al, developed a novel CMBO catalyst by blending hydrated lime and magnesium oxide (50:50) and subsequently calcining at 700 °C for 30 minutes.6 This CMBO catalyst was employed in the transesterification of jatropha curcas seed oil and methanol and evaluated the effect of the reaction temperature and time. Optimal conditions were identified, yielding 99% biodiesel in 60 minutes or 100% biodiesel in 90 minutes. Maimoonah and colleagues applied the hydration-dehydration technique to synthesize a series of nanostructured CMBO catalysts with varied molar ratios of two metals (ranging from 1:0.05 to 1:0.30).7 The catalyst was employed in sunflower oil-derived biodiesel synthesis via transesterification with methanol. The optimized conditions led to 97% biodiesel yield, achieved with a 25% molar ratio, atmospheric pressure, 3-hour reaction time, 65 °C reaction temperature, 5% catalyst loading, and a 9:1 methanol-to-oil molar ratio. Notably, the CMBO catalyst used in this report also showed significant promise for large-scale biodiesel production from sunflower oil.
Yan et al, reported the use of CMBO catalysts in the transesterification of rapeseed oil with methanol and discovered that the CMBO catalyst has better activity than pure CaO.8 These readily synthesized catalysts were optimized by changing carriers, Ca loading, and calcination temperatures. The conversion of rapeseed oil to 92% at 64.5 °C with the CMBO catalyst was achieved. Teo and colleagues investigated the synthesis of calcium-enriched CMBO using co-precipitation followed by thermal decomposition.9 Various mole ratios of Ca to Mg (Ca:Mg – 1:1, 1:2, 2:1) were explored to synthesize potential catalysts for effective biodiesel production. The inclusion of calcium aimed to enhance the basic nature of the CMBO system, thereby encouraging its performance in the transesterification of Elaeis guineensis oil with methanol. Using a 4 wt% catalyst loading, a 15:1 methanol-to-oil ratio, and a reaction time of 6 hours, it achieved a maximum of 99.0% FAME yield.
A report by Hu and coworkers described the synthesis of a series of CMBO catalysts through co-precipitation, by varying the Mg/Ca molar ratios (0:1, 1:3, 2:3, 1:1, 1:0) and applied them in the transesterification of soybean oil and methanol to disclose the impact of Ca: Mg ratios.10 The incorporation of Mg into the CMBO structure enhanced basic sites by reducing CaO lattice spacing, improving catalytic activity. However, it was also noticed that an excessive Mg content in CMBO led to detrimental effects on pore structure and specific surface area. The 1Mg3Ca catalyst, with higher Mg doping and optimal surface area (50.72 m2/g), performed well in the transesterification.
Buasria and team examined the natural dolomitic rock as an eco-friendly catalyst in microwave-assisted transesterification between Jatropha Curcas oil and methanol. The mineral rock, rich in CaMg(CO3)2, was calcined to produce a CMBO catalyst.11 They noticed an activation method can enhance the catalyst’s activity, basicity, and stability. The calcined dolomitic catalyst showed a 14.8 m2/g surface area. It was applied successfully as a catalyst in the transesterification of Jatropha Curcas oil with methanol. By varying reaction time, methanol/oil ratio, and catalyst loading, optimal conditions were determined. A conversion of 95% oil was obtained with activated dolomitic catalyst at 4 minutes reaction time, using 18:1 methanol/oil molar ratio, and 4 wt% catalyst loading. Taufiq et al also observed the efficacy of CMBO in the transesterification of Jatropha curcas oil with methanol and compared the activity with the single oxide counterparts.12 The CMBO was synthesized using a co-precipitation technique. The CaO-MgO demonstrated higher activity than CaO-ZnO in the transesterification of JCO. Under optimized transesterification conditions, the CMBO catalyst achieved JCO conversion rates exceeding 80%. The catalytic efficiency was attributed to the presence of robust basic sites on the surfaces, primarily associated with Ca2-O2 pairs. Abdulloh and colleagues used calcined dolomite for synthesizing biodiesel from tamanu oil.13 The catalyst’s basic strength was assessed using Hammett indicators, revealing a nanocrystal size of up to 52.99 nm. The optimal conditions for transesterification were realized at 65 °C, employing a methanol to oil molar ratio of 1:30, and a catalyst weight of 1g. Tamanu oil conversion into biodiesel was quantified using GC-MS, to obtain ~ 98% conversion.
Olivia and colleagues investigated the CMBO catalyzed trans-esterification of palm oil with methanol to produce biodiesel.14 They prepared a CMBO by subjecting dolomite powder to calcination for a duration of 3 hours at four distinct temperatures: 700 °C, 800 °C, 900 °C, and 1000 °C and observed that the CMBO catalyst obtained at 900°C, exhibited remarkable efficacy in facilitating the trans-esterification reaction between palm oil and methanol, resulting in biodiesel formation
A report by Abdelrahman et al described the transformation of waste cooking oil into biodiesel by employing calcined dolomite.15The catalytic performance of this CMBO catalyst exhibited remarkable ability, as evidenced by a notable biodiesel yield, reaching approximately 96.5%. This noteworthy outcome was achieved within a reaction duration of 120 minutes, utilizing a 6 wt% catalyst, a 1:15 oil/methanol ratio, and a reaction temperature of 90 °C. Tahvildari et al, reported the catalytic efficiency of a nano CMBO that was synthesized by a sol-gel method.16 Higher proportions of CaO to MgO were found to enhance the yield of biodiesel production from recycled cooking oil. Under the optimized conditions, the nano CMBO gave ~99% biodiesel yield.
A report by Lee and colleagues provided a comparison between CMBO and other non-noble metal oxide catalysts in the production of biodiesel from non-edible jatropha oils.17 Their study involved various solid base catalysts (CaO-MgO, CaO-ZnO, CaO-La2O3, and MgO-ZnO), prepared through co-precipitation and calcination at 800 °C for 6 hours. When these catalysts were employed in the transesterification of non-edible jatropha oil, the catalytic efficiency was found in the order of CaO-ZnO > CaO ~CaO-MgO ~CaO-La2O3 > MgO-ZnO > MgO > ZnO > La2O3. The study demonstrated the superior catalytic efficiency of CMBO catalysts. Overall, the CMBO catalysts achieved the highest catalytic activity (>90% biodiesel yield) with minimal metal leaching. Korbag and Korbag studied the transesterification of non-edible oils such as olive, sunflower, and corn in the production of biodiesel using CMBO.18 The catalysts were prepared by using commercial hydroxide samples that decomposed at 600 °C for 2h. The study focused on identifying optimal parameters, about a methanol to oil ratio of 6:1 and temperature range from 30 to 60 °C. Maximum biodiesel yield (99%) was obtained at 60 °C.
Abukhadra et al, investigated the catalytic efficiency of CMBO nanorods obtained via microwave irradiation19 in the trans-esterification of non-edible castor oil into biodiesel. The researchers did a comparison study with conventional and sonication procedures to determine their efficacy. The synthesized catalyst has a distinct rod-like shape and a large BET surface area of 112.8 m2/g. The researchers optimized the process under normal circumstances, reaching a biodiesel yield of 96.2% in 70 minutes. This was accomplished with a 6 wt% catalyst concentration, a 70 °C temperature, and a 15:1 molar ratio of ethanol to used castor oil. When the sonication process was used, the biodiesel production increased to ~99%.
Vahid and colleagues reported the synthesis of CMBO catalyst by co-precipitation with varying mass ratios of Ca:Mg (9:1, 8:2, 7:3, 6:4, and 5:5), followed by calcination at 700 °C for 3 hours.20 The CMBO displayed a combination of cubic CaO and hexagonal MgO phases. Adjusting the CaO:MgO ratio increased surface area and decreased pore diameter. Transesterification of n-butyl acetate from methanol experiment performed at 95 °C and atmospheric pressure, with the 8:2 CaO: MgO mass ratio in CMBO yielded ⁓83% conversion rate. This optimal performance was attributed to robust basic sites associated with Ca2+–O2− pairs. Calcined CMBO obtained at 900 °C has shown further enhancement in catalyst activity.
Albuquerque et al, studied the transesterification of ethyl butyrate with methanol using CMBOs with varied Mg: Ca ratios and compared the results with single oxide counterparts.21 A series of CMBOs were synthesized via the co-precipitation method by changing the Ca to Mg ratios. With a Mg: Ca molar ratio of 3, the CMBO is efficient in transesterification. This phenomenon was ascribed to the existence of potent basic sites on the catalyst surface, primarily linked to Ca2+–O2− pairs, as well as a significantly greater surface area compared to that of pure CaO. In contrast, MgO displayed inactivity in this process.
A comparative statement of the results and optimized conditions of various Transesterification reactions over CMBO catalyst is presented in Table 1.
Table 1: Comparative statement of transesterification over CMBO catalysts
| S.No | Oil | Alcohol:Oil | Cat. Wt% | Temp(°C) | Time | Yield% | Ref |
| 1 | Jatropa curcus | 5.5:1 | 1.5 | 60 | 90min | 100 | 6 |
| 2 | Veg. oil | 12:1 | 1.5 | 60 | 2h | 92.2 | 10 |
| 3 | n-butyl acetate | 5:1 | 0.1 | 95 | 2 | 95 | 20 |
| 4 | Sunflower | 9:1 | 5 | 65 | 3 | 97 | 7 |
| 5 | Jatropa | 25:1 | 3 | 120 | 3 | 94 | 17 |
| 6 | rapseed | 18:1 | 16.5 | 65 | 2 | 92 | 8 |
| 7 | Elacis guineensis | 15:1 | 4 | 60 | 6 | 99 | 9 |
| 8 | Waste cooking | 7:1 | 3 | 6 | 6 | 99 | 16 |
| 9 | Ethyl butarate | 4:1 | 3 | 60 | 1 | 100 | 21 |
| 10 | Soyabean | 7:1 | 4 | 65 | 2 | 100 | 14 |
| 11 | Jatropa curcus | 18:1 | 4 | 80 | 1 | 95 | 11 |
| 12 | Castor | 15:1 | 6 | 70 | 1.1 | 96.2 | 19 |
| 13 | Jatropa | 15:1 | 4 | 65 | 6 | 85 | 12 |
| 14 | Olive, sunflower, Corn | 6:1 | 2 | 60 | 4 | 99 | 18 |
| 15 | Lamann | 30:1 | 5 | 65 | 5 | 97.96 | 13 |
| 16 | Glycerol | – | 2 | 220 | 24 | 51 | 22 |
| 17 | Pulm | 11:1 | 15 | 60 | 1 | 78 | 15 |
Plausible reaction mechanism of transesterification: The transesterification of triglycerides in the presence of heterogeneous catalysts can follow either an Eley-Rideal or a Langmuir-Hinshelwood mechanism (Scheme 2). In step I, the alcohol undergoes activation at a surface basic site. This basic site, O2−, removes H+ from the alcohol, while the R–O– group (with R as a hydrocarbon chain) is adsorbed on a metallic site, forming an active anionic alkoxide and the adjacent metallic site adsorbs the carbonyl group, forming a cationic complex. This activation step is significant, especially in the presence of weak basic sites. Subsequently, in step II the activated alkoxide initiates a nucleophilic attack on the carbonyl group, leading to the addition of the alcohol chain to the hydrocarbon chain, thus creating a tetrahedral intermediate. This intermediate then obtains H+ from the surface basic site and undergoes rearrangement to produce the ester. In step III the organic molecule is then desorbed through the alignment of charges on the oxygen attached to the carbonyl group, resulting in the formation of an ester and a diglyceride. This stage holds particular significance for catalysts with robust basic sites. Additionally, this stage is repeated twice more, involving the diglyceride and the monoglyceride, leading to the production of three ester molecules and one glycerol molecule.
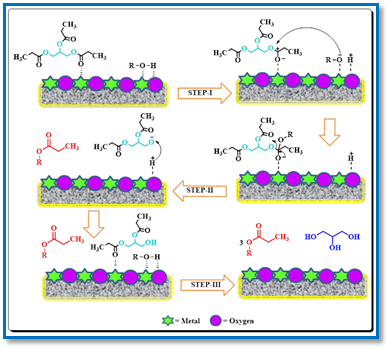
Scheme 2: CMBO catalyzed reaction mechanism of transesterification
- CMBO catalyzed C-C, C-heteroatom bond forming organic synthesis other than transesterification:
Literature survey reveals that the CMBOs efficiency is not limited to transesterification but is also suitable to perform a variety of C-C, C-heteroatom bond forming reactions of organic synthesis because of their solid-base nature. Suttibut et al, reported the application of CMBO catalyst in the isomerization of 1-butene. The CMBO catalyst was synthesized using co-precipitation.23 Varying CaO concentrations (0.19%, 1.77%, 2.40%, and 3.64% by weight) impacted the composition of CMBO (Scheme 3). The presence of CaO increased catalyst basic sites but decreased BET surface area due to reduced pore volume. The modified structure of CMBO improved OH− adsorption, enhancing catalytic activity. The CMBO catalyst with 1.77 wt% CaO displayed the highest performance and achieved 99% selectivity for 2-butene, superior to pure MgO and CaO catalysts.
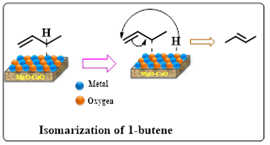
Scheme 3: CMBO catalyzed isomerization of 1-butene
Omata and colleagues employed a precipitation method to generate a CMBO catalyst and used it in methane oxidative coupling.24 The introduction of calcium ions (Ca2+) as dopants increased the catalyst’s basicity compared to pure MgO. This sharp basicity, particularly at 85% mol MgO, led to remarkable catalytic activity. Operating the reaction at 750 °C, the catalyst achieved a 9.2% methane conversion rate and 67.1% selectivity for C2 products (Scheme 4).
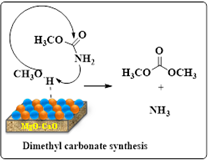
Scheme 4: Dimethyl carbamate synthesis via oxidative coupling of methane
A report by Vin’s group disclosed the preparation CMBO catalysts with varying CaO mass fractions (3.8%, 7.2%, 10.5%, 13.5%, and 16.3%) via the impregnation method.25 These catalysts were employed in an alcoholysis reaction to synthesize methyl formate from CO2-derived formamides and green methanol (Scheme 5). The catalyst with 13.5% CaO demonstrated greater performance to get 94.5% N-formylmorpholine conversion and 100% methyl formate selectivity at 120 °C. The study also established a direct correlation between strong basic site density and alcoholysis reaction efficiency, with higher base density resulting in greater reaction rates.
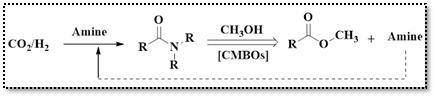
Scheme 5: Methyl formate synthesis from CO2
Alarcon et al, investigated the application of CaO, MgO, and a 10% CaO + 90% MgO composition in CMBO as catalysts for naphthalene steam gasification.26a,b Carbonate types varied, with CaO showing higher stability due to bidentate carbonates (Scheme 6). A synergistic effect on the 10% CaO + 90% MgO catalyst was attributed to cooperative action between the two oxides. The MgO was found to be responsible for the inhibition of bidentate carbonate formation and carbonaceous matter deposition on CaO surface, endorsing the creation of unidentate carbonates.
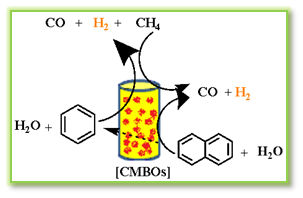
Scheme 6: Naphthalene steam gasification reaction for H2 production
Tang et al, demonstrated the CMBO efficacy in accelerating streptomycin hydrolysis degradation from waste water samples.27 When compared with homogeneous catalytic conditions, the performance of heterogeneous CMBO solid-base was found to be superior. The strong base sites present CMBO solid base surface interacts with the ether C-O bonds of streptomycin at two positions to produce four hydrolysis products and indicates the viability of improved hydrolysis. The metal-mediated hydrolysis pathway of streptomycin is depicted in the following Scheme 7.
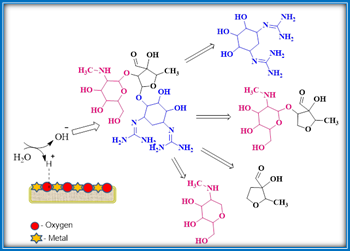
Scheme 7: Hydrolysis of streptomycin mediated by CMBO
Taralas et al, reported about non-catalytic and catalytic cracking of n-heptane in the presence of CaO, MgO, and Calcined Dolomites i.e., CMBO sample.28 The overall thermal cracking of n-heptane was compared to the cracking in the presence of the basic catalysts MgO, CaO (low surface quicklime), and CMBO. The dolomite effectively catalyzed the water-gas reaction than the single oxide counterpart (Scheme 8).
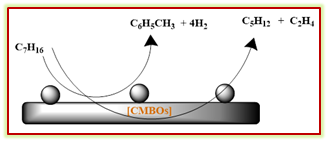
Scheme 8: Catalytic cracking of n-heptane
Philipp and colleagues synthesized CMBO catalysts with varying mole percentages of MgO/CaO, specifically 85%, 75%, 50%, and 25%, and studied effectiveness as catalysts in the oxidative coupling of methane.29 The best results, with maximum selectivity (67%) and activity were achieved with the 85% CMBO catalyst at 1023 K. The incorporation of CaO into MgO enhanced the basicity of the catalyst, which was associated with changes in surface morphology. Additionally, the surfaces of the MgO-rich oxides were predominantly covered by MgO (Scheme 9a).
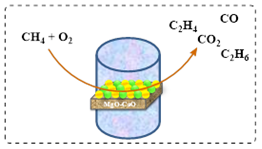
Scheme 9a: Oxidative coupling of methane reaction
Dang’s group studied the CMBO catalysts with varying Mg/Ca ratios (0.5, 1, 2) for propylene carbonate (PC) synthesis from urea and propylene glycol.30 The catalysts were obtained using different precipitating agents (NaOH, Na2CO3). The most effective catalyst had a 1:1 Mg/Ca ratio, synthesized with Na2CO3, yielding 96% PC, 99% selectivity, and 96% urea conversion at 160 °C. FE-SEM analysis revealed the presence of small MgO particles and large CaO aggregates on the catalyst’s surface (Scheme 9b).
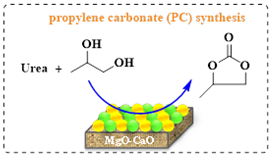
Scheme 9b: PC synthesis reaction over CMBO
Tamaddon and colleagues reported the application of natural Dolomite (CaMg(CO3)2) mineral, a precursor for CaO-MgO, as a recyclable natural catalyst in Henry, Knoevenagel, and Michael reactions in water for the preparation of nitroalkanols, trisubstituted alkenes, b-amino and b-thio substituted carbonyl compounds.31 (Scheme 10 a & b) This heterogeneous catalyst has been recently used in the production of biodiesels via the transesterification reaction of canola oil with methanol. The water-insoluble catalyst Iranian dolomite was characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), Brunauer Emmett Teller (BET), and XRF chemical analysis. The basic strength of the catalyst was evaluated by Alam following the Hammett indicators procedure. Structure-activity relation between the Dolomite catalyst and the substrates of the abovementioned three reactions was deduced.
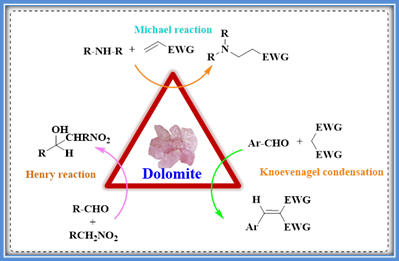
Scheme 10a: Dolomite catalyzed C-C and C-hetero atom bond forming reactions
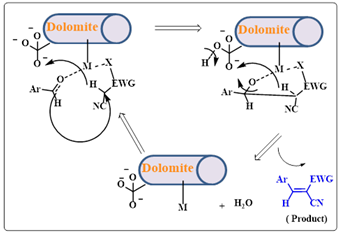
Scheme 10b:Dolomite catalyzed reaction mechanism of Knoevenagel condensation reactions Alam et al, investigated a valorization route for 6-amyl-α-pyrone (6PP) by conducting the aldol condensation reaction of nonanones with furfural and HMF.32 The reaction was performed under neat conditions without the requirement of an organic solvent. A mixed oxide catalyst of Ca and Mg i.e., CMBO was utilized for gaining higher yields of the aldol products. The liquid aldol products may directly undergo catalytic upgrading via hydrodeoxygenation (HDO) to yield branched C14 or C15 alkanes to be used as diesel and jet range fuels (Scheme 11).
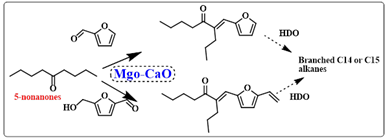
Scheme 11: CMBO catalyzed hydrodeoxygenation
Jose et al studied the production of glycerol oligomers using natural dolomite and calcinated dolomite.33 The calcinated dolomite exhibited a decrease in the particle size (297 to 153 nm) and improved the surface area (1.57 to 37.7 m2/g) and basic strength. Calcined dolomite showed better catalytic performance than natural dolomite during the oligomerization in terms of glycerol conversion and selectivities for diglycerol and triglycerol. The interaction between the catalyst and the substrates in catalyzing the oligomerization is depicted in Scheme 12.
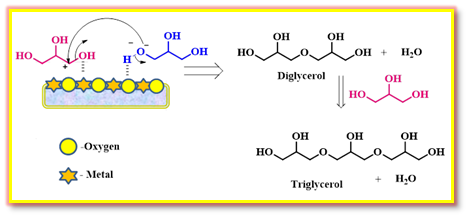
Scheme 12: Glycerol oligomerization
Yang et al, reported the efficiency of calcined Dolomite as an efficient and recyclable catalyst for Knoevenagel condensation reaction for the synthesis of α,β-unsaturated carbonyl compounds.34 The Knoevenagel condensation of aldehydes with active methylene compounds such as malononitrile and ethyl cyanoacetate was studied and optimized in the presence of calcined Dolomite i.e., CaO-MgO based CMBO. The authors mentioned that calcination temperature is an important factor that changed the surface areas, textural characteristics, and basicity of calcined dolomite catalysts. The authors identified that among calcined dolomite catalyst samples, the one calcined at 700 °C served as the best catalyst for this reaction. Reaction parameters such as the effect of solvent, catalyst amount, and catalyst basicity were assessed. The model reaction was also checked in the presence of natural dolomite and recorded longer reaction times as compared to the calcined dolomite. Further, it was also recognized that the calcined catalyst is recyclable four times without a significant decrease in reactivity (Scheme 13 a & b).
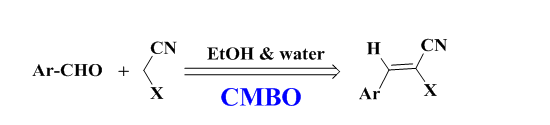
Scheme 13a: CMBO catalyzed Knoevenagel condensation reaction
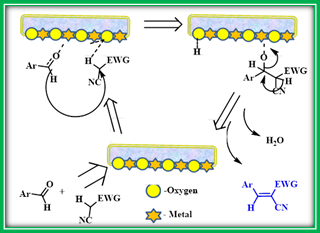
Scheme 13b: Knoevenagel condensation mechanism catalyzed by CMBO
Bandalla et al, synthesized CaO–MgO binary metal oxides (CMBOs) using the co-precipitation method with different mass ratios and used them as efficient and reusable heterogeneous catalysts for the selective oxidation of cyclohexanol to cyclohexanone under solventless condition.4 The 1CaO–1.5MgO catalyst composition displayed a higher conversion of cyclohexanol (~85%) with superior selectivity toward cyclohexanone product (~92%) at 140 °C. The basic sites have played a key role in the alcohol deprotonation and activation of the C–H bond on the associated α–carbon of the alcohol (Scheme 14).
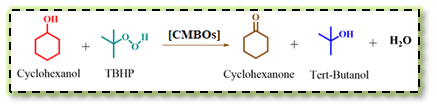
Scheme 14: Selective oxidation of cyclohexanol to cyclohexanone
Yan and colleagues used a CMBO catalyst in the degradation of lignin.35 The catalyst was prepared by impregnating Ca(CH3COO)2 on a MgO carrier followed calcination at 700 °C. The catalyst exhibited high surface basicity (30.2 mmol/g) when the CaO/MgO ratio in CMBO was 0.08 (Scheme 15). During the catalytic degradation of lignin, there was a substantial reduction in the lignin’s average molecular weight (from 3,000 to under 800) and a notable increase in hydroxyl content (from 200 to over 500). FT-IR analysis revealed a reduction in ether bonds and a significant increase in hydroxyl bonds.
Scheme 15: Lignin degradation using CMBO
The use of CaO-MgO solid base catalyst to synthesize vinyl methyl ether (VME) from biomass-derived ethylene glycol dimethyl ether was investigated by He and colleagues.36 It is known that vinyl methyl ether is an important intermediate to obtain functional polymers and fine chemicals (Scheme 16). Generally, Reppe vinylation is followed in industry to obtain VME, which involvestheaddition of methanol to acetylene using high pressures and caustic bases as catalysts and limits its applications. Hence, biomass-derived ethylene glycol dimethyl ether (EGDE) via the methanol elimination reaction catalyzed by CaO-MgO solid bases at atmospheric pressure was established for the sustainable synthesis of VME. The CMBO synthesized via the co-precipitation method with a Ca/Mg molar ratio of 2 exhibited high efficiency and stability to get 100% EGDE conversion and 93.5% selectivity to VME at 400 °C. The authors explained that the CMBO catalyst surface possesses strongly basic sites and facilitates the formation of VME.
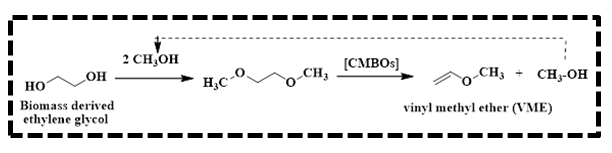
Scheme 16: Synthesis of vinyl methyl ether (VME)
Elumalai’s research group described about the catalytic application of MgO/CaO based CMBO nanocomposite for the profitable generation of D-fructose and D-allulose from glucose in aqueous media.37 The CMBO with equal proportions of MgO and CaO helped to manipulate the strong basicity and surface properties. Further, the presence of MgO at the surface initiates the isomerization reaction by providing a higher number of weak/medium base sites. It was also proposed that the CaO present beneath begins the sequential conversion of the enol-intermediate to ultimate fructose and byproducts (mannose and allulose). Thus, the CMBO catalyst accelerated the glucose interconversion to get fructose with higher yields and selectivity in shorter reaction times. Besides, it also initiated the C-3 fructose epimerization to yield allulose which is a low-calorie sugar molecule (Scheme 17).
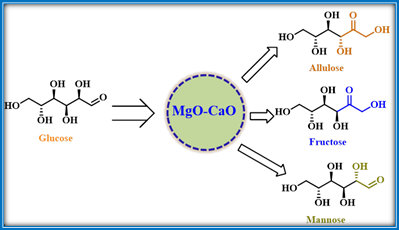
Scheme 17: CMBO catalyzed generation of D-fructose and D-allulose from glucose
Acetylation of glycerol using CMBO catalyst was reported by Ramirez and colleagues.38 Simple co-precipitation method, with varying CaO compositions of 20%, 30%, and 50% by weight was used to prepare the CMBO catalyst. A CMBO with an equal mix of 50% MgO and 50% CaO displayed the highest glycerol conversion rate and lowest production of diacetin and monoacetin (Scheme 18).
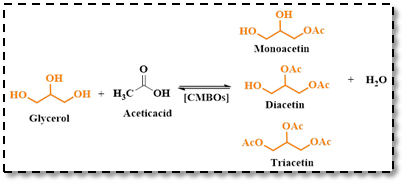
Scheme 18: Glycerol acetylation
Delgado and Aznar investigated the effectiveness of CaO, MgO, and a CMBO (calcined dolomite) in hot gas from biomass gasification with steam was explored.39 Calcined dolomite outperformed pure oxides due to its relatively larger surface area (11.2 m2/g, 5.4 and 9.5 m2/g for CaO and MgO, respectively). Shahid et al, studied methylene blue degradation using CaO and CaO-MgO catalysts. They explored various preparation methods, including conventional heating and hydrothermal processes.40 Higher temperatures (up to 160 °C) led to increased catalytic efficiency (0.315 min⁻¹), but excessive heat caused particle aggregation, reducing surface area. CaO performed better in low dielectric constant solvents, while CaO/MgO based CMBO showed the opposite trend.
- CMBO assisted Sorption studies
Fossil fuel combustion is a significant contributor to dangerous CO2 emissions, which have negative effects on the environment and the economy. Carbon capture, utilization, and storage (CCUS) is crucial in order to fight this. It is essential to create solid sorbents with high absorption capacities, rapid reactivity, stability, low decomposition temperatures, and quick rates at high temperatures. Due to their affordability, high CO2 absorption capability, and quick chemical reactivity, magnesium oxide (MgO) and calcium oxide (CaO) are intriguing choices (Figure 4).
Park and Yi investigated the impact of preparation methods on CaO-based CO2 adsorbents.41 They compared co-precipitation and hydration methods at different CaO-MgO ratios. Co-precipitated samples displayed excellent cyclic stability but low CO2 absorption capacity. An additional hydration step improved CO2 pathways and dispersion, significantly boosting absorption capacity. In particular, Ca75Mg25, following the hydration step, achieved a remarkable absorption capacity of 43.5 g CO2/g absorbent in a multi-cycle test.
Nethravathi and colleagues explained the use of porous CaO–MgO based CMBO nanostructures for CO2 capture.42 The CMBO porous nanostructures with varied amounts of MgO (10–40 mol %) exhibit CO2 capture capacities of 67–51 mass % of the sorbent. CaO80–MgO20 porous nanostructures captured 61.6 mass % of CO2 and retained 84.6% (52.1 mass % of CO2) of its initial capacity after 100 carbonation–decarbonation cycles. The CMBO porous nanostructures exhibit improved cycle stability in addition to high CO2 capture capacity. The high carbonation efficiency and cycling stability of the porous nanostructures as CO2 sorbents are attributed to the synergistic combination of a large surface area, a porous network, and an inert MgO stabilizer.
Lan´s research group studied the effect of porous nanostructured CMBO based adsorbents for the purpose of CO2 adsorption uptake.43 It was observed that as the MgO content increased in the BMO, both the specific surface area and pore radius also increased and the higher concentration of MgO had a positive impact on the reaction rate and the overall durability of the nano-CaO/MgO-based adsorbent. Notably, these adsorbents demonstrated exceptional durability, showing high conversion rates not only at low calcination temperatures in the absence of CO2 but also at elevated temperatures in the presence of high concentrations of CO2.
Yan and colleagues developed a novel CO2 sorbent composed of both CaO and MgO i.e., BMO obtained from calcined dolomite.44 The study investigated the influence of several factors, including the mass ratio of carbide slag to dolomite, calcination conditions, and the presence of steam during carbonation, on the CO2 capture performance of this novel CaO/MgO sorbent over multiple calcium looping cycles. Notably, the sorbent exhibited its highest CO2 capture capacity when the mass ratio of carbide slag to dolomite was set at 74:26, resulting in a CaO/MgO sorbent with a mass ratio of CaO to MgO of 90:10.
A report by Al-Awaji and team described the synthesis of CMBO by sol-gel method, incorporating gum arabic extract, and evaluated its efficiency as sorbent in removing cobalt ions (Co (II)) from aqueous solutions.45 The resulting CMBO composite is featured with a surface area (50 m2/g), a pore size (11.79 nm), and a porous nanostructure comprising irregular spherical particles (20 nm in size). The Co (II) adsorption followed Langmuir adsorption isotherm and displayed a removal capacity of 469.5 mg/g. This report further highlights the removal of other heavy metal ions and organic pollutants.
Olivas et al, reported the experimental evaluation of hydrogen production via improved ethanol steam reforming combined with CO2 absorption using CaO, CaO-MgO based CMBO based solid adsorbents in the presence of Ni/Al2O3 catalyst.46 The authors made an analysis that renewable energy sources (hydro, geothermal, solar, wind, biomass, biofuels, etc.) have grown rapidly in recent times and stated that conventional energy from fossil remains predominant. Therefore, the development of clean energy sources like hydrogen energy is the current challenge. In this reported system, using ethanol as a feedstock combined with the in-situ absorption of CO2 is highly attractive for the production of H2 which would be suitable to be used as a feed gas in hydrogen fuel cells.
Zhu and team introduced a novel particle model for the absorption capacity of CaO, comparing pure CaO precursors and CMBO mixtures synthesized via ball milling.47 An effort was made to relate CO2 uptake to factors like pore structure, surface area, particle radius (r), and inter-particle distance after carbonation. Inter-particle distance was found to be a key factor for adsorption capacity. The CaO derived from D-gluconic acid calcium salt monohydrate achieved 80% conversion with minimal loss (0.344%) over cycles, while the 1:1 CaO-MgO catalyst exhibited 100% conversion and high stability (only 3.9% loss after 100 carbonation-calcination cycles).
Diana and the team produced sorbents by co-precipitating calcium oxide (CaO) with varying magnesium oxide (MgO) levels (5% to 30%).48 The addition of MgO reduced sintering effects in CaO sorbents. Sorbents with 5% Ca and 10% MgO maintained CO2 adsorption capacity through multiple cycles. Notably, CaO with 10% MgO consistently adsorbed CO2 over 30 carbonation cycles, indicating enhanced performance, structural stability, and surface area.
Yan et al, introduced a novel CaO-MgO material as CO2 sorbent was created from carbide slag and dolomite via the combustion method in the calcium looping process.49 The carbide slag serves as a CaO precursor, while dolomite contributes to both MgO and CaO. Optimal results were achieved with a 74:26 mass ratio of carbide slag to dolomite, yielding a CMBO sorbent with a 90:10 mass ratio. This sorbent exhibited excellent CO2 capture capacity, retaining 0.52 g CO2 per gram of sorbent after 20 cycles. It outperformed sorbents made from analytical reagents and showed increased capacity when calcined in pure steam at 800 °C, making it a promising option for the calcium looping process in CO2 capture.
AbuKhadra et al, reported the synthesis of new CMBO based nanorods on diatomite frustules by combining hydrothermal synthesis with microwave irradiation.50 Pharmaceutical residues from levofloxacin were successfully eliminated by this substance. The CMBOs featured rod-shaped nanoparticles with a size of 52.3 nm and a surface area of 112.8 m2/g BET. The ideal circumstances led to theoretical levofloxacin absorption of 106.7 mg/g, with an equilibrium time of 720 minutes at pH 7. CMBO Nano rod catalysts prepared via hydrothermal synthesis showed better adsorption performance than CMBO catalysts made via precipitation.
Ahmed et al, reported a template-free method to create hierarchically porous CMBOs and their adsorption performance for phosphate and methyl orange (MO).51 As the Mg2+/NH3 feeding ratio increased, the average pore size and porosity decreased. Among the samples, MgO-25 displayed the highest surface area (63 m²/g by mercury intrusion) while MgO-50 exhibited the highest BET surface area (121 m²/g). All MgO samples followed pseudo second-order kinetics for phosphate adsorption and pseudo second-order and Freundlich isotherms for MO adsorption. MgO-25 demonstrated the most significant phosphate removal capacity at 478.5 mg/g and the highest MO removal capacity at 4483.9 mg/g among all investigated samples (Figure 5).
Conclusions: The literature survey reveals that CMBOs (CaO-MgO) have a good capacity to serve as the main catalyst in many heterogeneous catalytic processes related to sustainable organic and inorganic chemistry synthesis. The reported structural analysis of these CMBOs indicates their aptness in catalyzing a variety of C-C, C-N, and C-S bond forming reactions as well as important inorganic conversions. It is now understood that these CMBOs are no longer considered just as catalyst supports but also as efficient main catalysts. Moreover, these CMBOs are easy to synthesize from earth-abundant and inexpensive alkaline earth metal precursors, thermally stable, contain an ample number of basic sites, and are eco-compatible. The reaction mechanisms proposed for CMBO catalytic cycles of various organic syntheses indicate the information about suitable active sites and promote the scope for many other relevant reactions. Besides, these CMBOs are also recognized as good sorbents in industrial and environmental processes. It is always not necessary to combine other non-noble or noble metal catalyst materials with CMBOs.
References:
1 W. Bing and M. Wei, Journal of Solid State Chemistry, 2019, 269, 184–194.
2 S. Dresp and P. Strasser, ChemCatChem, 2018, 10, 4162–4171.
3 A. I. Tsiotsias, N. D. Charisiou, I. V. Yentekakis and M. A. Goula, Catalysts, 2020, 10, 36.
4 J. A. Tavizón-Pozos, G. Chavez-Esquivel, V. A. Suárez-Toriello, C. E. Santolalla-Vargas, O. A. Luévano-Rivas, O. U. Valdés-Martínez, A. Talavera-López and J. A. Rodriguez, Energies, 2021, 14, 1031.
5 S. Bandalla, V. Dosarapu, G. B. Bathula, M. Ravula, J. Yadagiri, P. Gogoi, M. Baithy, S. B. Jonnalagadda and C. S. Vasam, Molecular Catalysis, 2022, 533, 112759.
6 H. Ibrahim, D. C. U. Nwakuba, G. Abubakar and B. E. Nwobi, Asian Journal of Engineering and Technology, 2013, 01, 2321–2462.
7 M. K. Qasim, Egyptian Journal of Chemistry, 2019, 62, 475–485.
8 S. Yan, H. Lu and B. Liang, Energy and Fuels, 2008, 22, 646–651.
9 S. H. Teo, U. Rashid, S. Y. Thomas Choong and Y. H. Taufiq-Yap, Energy Conversion and Management, 2017, 141, 20–27.
10 M. Hu, J. Pu, E. W. Qian and H. Wang, Bioenergy Research, 2013, 1, 13. DOI:10.1007/s12155-023-10580-z.
11 A. Buasri, K. Rochanakit, W. Wongvitvichot, U. Masa-Ard and V. Loryuenyong, Energy procedia, 2015, 79, 562-566.
12 Y. H. Taufiq-Yap, H. V. Lee, M. Z. Hussein and R. Yunus, Biomass and Bioenergy, 2011, 35, 827–834.
13 A. Abdulloh, A. A. Widati and O. Arizal, Journal of Chemical Technology and Metallurgy, 2017, 52, 1150–1156.
14 R. Olivia, N. Jamarun, S. Arif and Y. A. Sirin, Rasayan Journal of Chemistry, 2017, 10, 160–164.
15 A. M. Rabie, M. Shaban, M. R. Abukhadra, R. Hosny, S. A. Ahmed and N. A. Negm, Journal of Molecular Liquids, 2019, 279, 224–231.
16 K. Tahvildari, Y. N. Anaraki, R. Fazaeli, S. Mirpanji and E. Delrish, Journal of Environmental Health Science and Engineering, 2015, 13, 1–9.
17 H. V. Lee, J. C. Juan, T. Y. Yun Hin and H. C. Ong, Energies, 2016, 9, 611.
18 S. Korbag and I. Korbag, Eurasian Chemical Communications, 2023, 5, 382–391.
19 M. R. Abukhadra, A. S. Mohamed, A. M. El-Sherbeeny, A. T. A. Soliman and A. E. E. Abd Elgawad, Chemical Engineering and Processing – Process Intensification, 2020, 154, 108024.
20 V. Mahdavi and F. Abedini, Chemical Engineering Communications, 2016, 203, 114–122.
21 M. C. G. Albuquerque, D. C. S. Azevedo, C. L. Cavalcante, J. Santamaría-González, J. M. Mérida-Robles, R. Moreno-Tost, E. Rodríguez-Castellón, A. Jiménez-López and P. Maireles-Torres, Journal of Molecular Catalysis A: Chemical, 2009, 300, 19–24.
22 F. J. S. Barros, J. A. Cecilia, R. Moreno-Tost, M. F. de Oliveira, E. Rodríguez-Castellón, F. M. T. Luna and R. S. Vieira, Waste and Biomass Valorization, 2020, 11, 1499–1512.
23 P. Suttibut, K. Suriye, P. Praserthdam and J. Panpranot, Journal of Nanoscience and Nanotechnology, 2017, 18, 439–444.
24 K. omata, A. Aoki and Fulimoto, Catalysis Letters, 1990, 4, 241–244.
25 X. He, X. Guo, G. Xia, R. Xu, Y. Wu and X. Luan, Catalysts, 2023, 13, 487.
26 a) N. Alarcón, X. García, M. Centeno, P. Ruiz and A. Gordon, Surface and Interface Analysis, 2001, 31, 1031–1041; b) N. Alarcón, X. Garcı́a, M. A. Centeno, P. Ruiz, A. Gordon, Applied Catalysis A: General,2004, 267, 251-265.
27 M. Tang, X. Dou, Z. Tian, M. Yang and Y. Zhang, Chemical engeneering journal, 2019, 355, 586-593.
28 G. Taralas, V. Vassilatos, K. Sjöström and J. Delgado, The Canadian Journal of Chemical Engineering, 1991, 69, 1413–1419.
29 R. Philipp, K. Omata, A. Aoki and K. Fujimoto, Journal of Catalysis, 1992, 134, 422–433.
30 K. Dang, N. Kumar, V. C. Srivastava, J. Park and M. Naushad, Materials, 2023, 16, 735.
31 F. Tamaddon, M. Tayefi, E. Hosseini and E. Zare, Journal of Molecular Catalysis A: Chemical, 2013, 366, 36–42.
32 M. I. Alam, S. Gupta, A. Bohre, E. Ahmad, T. S. Khan, B. Saha and M. A. Haider, Green Chemistry, 2016, 18, 6431–6435.
33 F. J. S. Barros, J. A. Cecilia, R. Moreno-Tost, M. F. de Oliveira, E. Rodríguez-Castellón, F. M. T. Luna and R. S. Vieira, Waste and Biomass Valorization, 2020, 11, 1499–1512.
34 H. Yang, H. Dong, T. Zhang, Q. Zhang, G. Zhang, P. Wang and Q. Liu, Catalysis Letters, 2019, 149, 778–787.
35 W. Yan, C. Yong-mei, W. Ping-yu, H. Yan-ming, Q. Te-fu, Chemistry and Industry of forest products, 2012, 32, 81-86.J
36 X. He, X. Guo, G. Xia, R. Xu, Y. Wu and X. Luan, Catalysts, 2023, 13, 487.
37. S. M. Arumugam, D. Singh, S. Mahala, B.Devi, S. Kumar, S. Jakhu, and S. Elumalai, Industrial Engineering chemistry research, 2022, 61, 2524-2537.
38 M. E. M Ramirez, I. Elizalde, R. López, M. T. Valdez,M. E. Reaction Kinetics, Mechanisms and Catalysis, 202,. 130, 417-431.
39 Delgado, M. P. Aznar and J. Corella, Industrial and Engineering Chemistry Research, 1997, 36, 1535–1543..
40 M. Shahid, M. A. Farrukh, A. A. Umar and M. Khaleeq-Ur-Rahman, Russian Journal of Physical Chemistry A, 2014, 88, 836–844.
41 J. Park and K. B. Yi, International Journal of Hydrogen Energy, 2012, 37, 95–102.
42 R. Rajamathi, Bojraj and C. Nethravathi, ACS Appl. Nano Mater, 2021, 4, 10969-10975
43 P. Lan and S. Wu, Chemical Engineering and Technology, 2014, 37, 580–586.
44 X. Yan, Y. Li, X. Ma, J. Zhao, Z. Wang and H. Liu, New Journal of Chemistry, 2019, 43, 5116–5125.
45 N. Al-Awaji, M. Boukriba, H. Idriss, M. A. Ben Aissa, M. Bououdina and A. Modwi, Advances in Materials Science and Engineering, 2022, 12.
46 D. Y. A. Olivas, M. R. B. Guerrero, M. A. E. Bretado, M. M. D. Paula, J. S. Gutiérrez, V. G. Velderrain, A. L. Ortiz and V. C.-Martínez, International Journal of Hydrogen Energy, 2014, 39, 16595–16607.
47 Q. Zhu, S. Zeng and Y. Yu, Environmental Science and Technology, 2017, 51, 552–559.
48 F. Diana, K. Vignesh, S. Sreekantan, A. Rahman Bin, New Journal of Chemistry, 2015, 40, 1–11.
49 X. Yan, Y. Li, X. Ma, J. Zhao, Z. Wang and H. Liu, New Journal of Chemistry, 2019, 43, 5116–5125.
50 M. R. AbuKhadra, M. G. Basyouny, A. A. AlHammadi, A. M. El-Sherbeeny and M. A. Salam, Scientific Reports, 2020, 10, 1–11.
51 S. Ahmed, Y. Guo, D. Li, P. Tang and Y. Feng, Environmental Science and Pollution Research, 2018, 25, 24907–24916.
