Halogenated aromatic compounds are of considerable commercial importance in organic synthesis. These compounds are potential disinfectant1, antiseptic2 and Fungicides3 and key precursors in the synthesis of organometallic regents4-7 and coupling reactions8.
Halogenation of aromatic substrates are electrophilic aromatic substitution reaction9-10. Among halogenation of aromatic substrates, bromination is the fastest followed by chlorination, iodination is the slowest11.
In the past, molecular bromine was commonly used as a brominating agent12-13. But this reagent is toxic, hazardous and releases corrosive hydrogen bromide as reaction side product. If carelessly used, it may cause skin burn. To avoid these problems, bromate-bromide reagent is used for the in-situ generation of bromine for bromination. However, this mixture also produces hydrogen bromide as laboratory waste. To overcome above difficulties researchers had diverted their attention on N-halocompounds14-16 for halogenation. In last few decades N-bromosuccimide17-20, N-bromoacetamide and N-bromopthalimide21 are used as brominating agents. In recent past N-bromopthalimide is preferred over N-bromosuccinimide due to its more stability.
The quantitative assessment of relative reactivity of bromination of regioisomers of cresol by N-bromopthalimide in aqueous solution has been found lacking behind presumably due to the rapidity of these reactions. Therfore, it is necessary to use some advance technique for study of these reactions.
In present study, we have studied the kinetics of bromination of regioisomers of cresol by N-bromopthalimide in aqueous solution using (2.0 x 10-3M) potassium chloride as supporting electrolyte using rotating platinum electrode techniques we have quantitatively assessed the reactivity of o-, m- and p-cresol using N-bromopthalimide as brominating agent.
In these reactions, only N-bromopthalimide is the electroreducible species among the reactants and products. Hence, the reactions can be followed by measuring the decrease in diffusion current due to N-bromopthalimide in the reaction, at a rotating platinum electrode.
MATERIALS AND METHODS
All the chemicals used in the present study were of analytial grade. Doubly distilled water was used to prepare all the solutions. Stock of solutions of 1.0 x 10-3M, O-cresol, m-cresol, p-cresol N-bromopthalimide supporting electrolyte 1.0 x 10-1M potassiumchloride were prepared in doubly distilled water. Citrate buffer of pH 6 was prepared by taking 24-209g of sodium citrate dihydrate +192.12g of citric acid +800ml water, the solution prepared by dissolving and then pH 6 was adjusted with the help of 0.1N HCl. Finally, the solution is diluted to 1 litre.
Electrodes
Cathode : Rotating platinum electrode
Anode : Saturated calomel electrode
Calibration
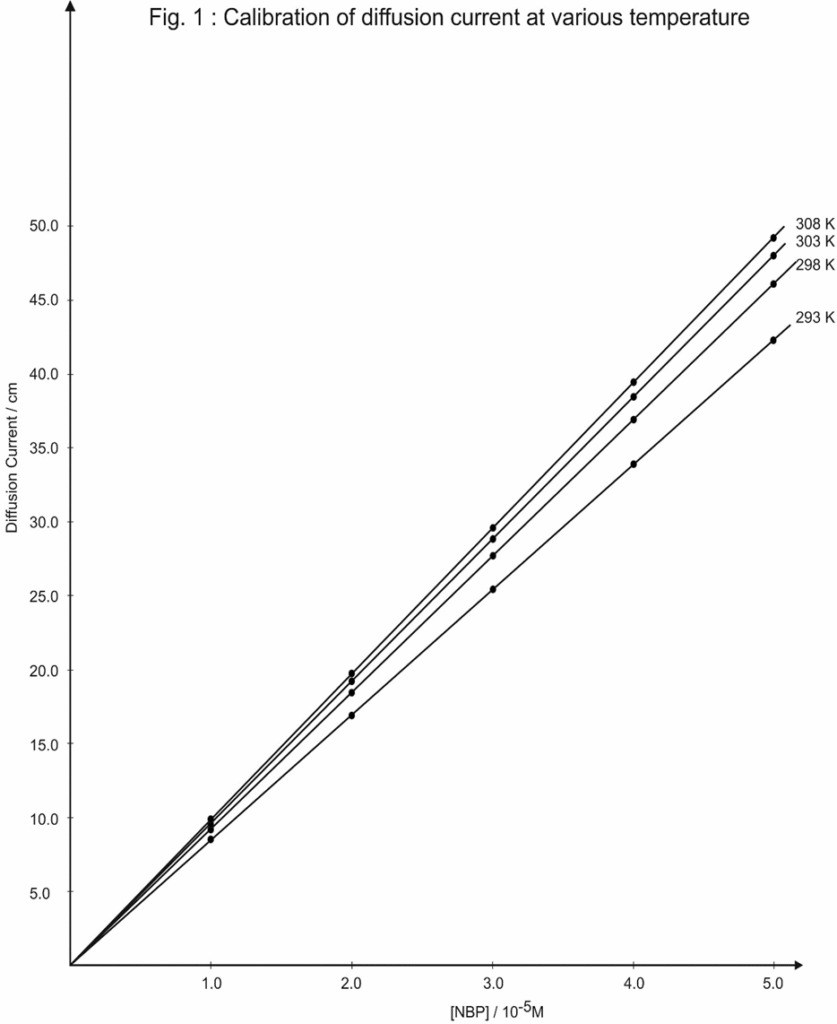
Saturated calomel electrode and rotating platinum electrode are dipped in 50.0cm3 of 2.0 x 10-3 M potassium chloride supporting electrolyte. A potential of 0.1 v was applied against saturated calomel electrode at rotating platinum electrode. The light spot of galvanometer was adjusted to zero scale. The supporting electrolyte was then replaced by 5.0*10-5M N-bromothalimide solution containing hundred fold supporting electrolyte. Shunt was adjusted for deflection of galvanometer spot to around 50 cm. The shunt kept undisturb through out the study. The diffusion current (galvanometer deflection in cm) values, was recorded for various concentrations of N-bromophtalimide in range of 1.0 x 10-5M to 5.0 x 10-5 M. A typical plot of deflection of galvanometer in cm (diffusion current) was plotted against the concentration of N-bromopthalimide similar determinations were made at various temperature (Table 1 and Fig.1).
Table 1 : Calibration of diffusion current. Current at various temperature.
| [NBP]10-5 M | Diffusion current / hA | |||
| 20 | 25 | 30 | 35 | |
| 1.0 | 9.0 | 9.5 | 10.0 | 10.5 |
| 2.0 | 17.5 | 18.5 | 19.5 | 20.5 |
| 3.0 | 26.0 | 27.5 | 29.0 | 30.5 |
| 4.0 | 34.5 | 36.5 | 39.0 | 41.5 |
| 5.0 | 43.0 | 45.5 | 48.0 | 49.5 |
Kinetic study
50.0cm3 of 5.0 x 10-5M o-cresol solution containing 2.0 x 10-3 M potassium chloride and 50.0cm3 of 5.0 x 10-5 M N-bromopthalimide containing 2.0 x 10-3M potassium chloride containing required quantity of citrate buffer solution were kept in thermostat at desired temperature in two different flasks. When the solutions has attaineded the desire temperature, the two solution. were mixed in reaction vessel kept in the thermostat in which the SCE was dipped and rotation platinum electrode was rotating. Stop clock was suddenly started at the time of mixing of solution. The galvanometer deflection reading was recorded at every 20 seconds. From the diffusion current reading the concentration of unreacted N-bromopthalimide (a-x) at various instant of time were obtained from calibration curve.
A plot of 1/(a-x) versus time was found to be linear (Table 2 and Fig.2). The slop of the spot gives the specific reaction rate, K. Similar kinetic study were carried out at various temperature ranging from 20.00c to 35.00c. From these studies, energy of activation (Ea), frequency faetor (A), and entropy of activation (Ds#) were evaluated (Table 3,4 and Fig. 3)
Similar experiments were carried out for m-cresol and p-cresol.
Table 2 : Kinetics of bromination of o-cresol by N-bromopthalimide in aqueous solution at 25.00C
| Time/s | Diffusion current / hA | [NBP]/10-5M | [NBP]/104M-1 |
| 0 | 45.5 | 5.00 | 2.00 |
| 20 | 38.7 | 4.25 | 2.35 |
| 40 | 28.6 | 3.70 | 2.70 |
| 60 | 18.8 | 3.28 | 3.05 |
| 80 | 11.0 | 2.94 | 3.40 |
| 100 | 5.9 | 2.67 | 3.75 |
| 120 | 2.9 | 2.44 | 4.10 |
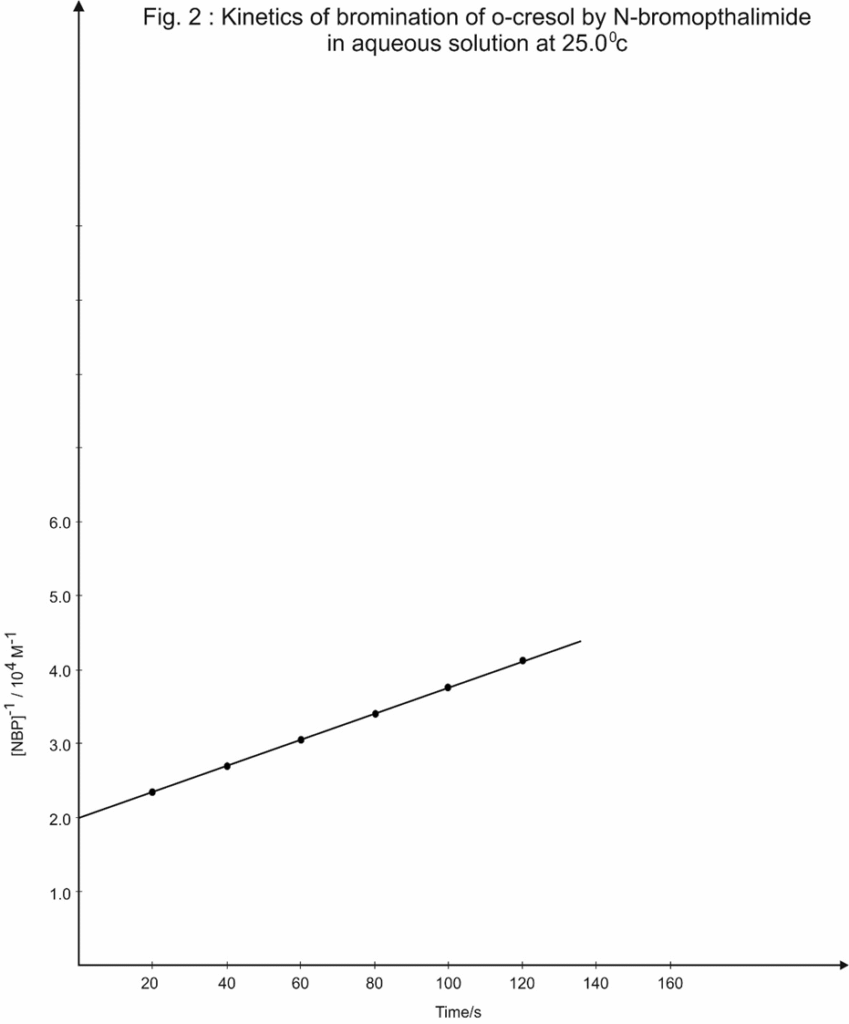
Result and Discussion
In present study one mole of each isomer of creasol reacts with one mole of N-bromopthalimide and give respective bromocresol as product. Hence the overall reactions are second order. The linear plots are obtained for reciprocal concentration of N-Bromopthalimide against time in each case (Table 2 and Figure 2) confirmed the second order kinetics.
Thus, the rate of these reactions are given as :
Rate = k2 [cresol] [N-Bromopthalimide]
Where, k2 is the second order rate constant, which is where, k2 is obtained from the slope of the above plot. The value of rate constant obtained in case of o-cresol, m-cresol and p-cresol are 175 M-1S-1, 7398 M-1S-1 and 410 M-1S-1 respectively at 25.00c.
The activation energy, Ea was calculated by plotting the log k2 against 1/T using Arrhenious equation;
lnk = ln A – Ea/RT
Where, k is the rate constant of reaction, A is the Arrhenious constant, T the absolute temperature and R is the universal gas constant.
The Arrhenious constant, A was calculated using the equation;
k = A e -Ea/RT
Where; k is the rate constant of reaction, A is Arrhenious constant, Ea is the activation energy, R is universal gas constant and T is absolute temperature.
The thermodynamic parameter, activation entrophy (∆S≠) was calculated using Erying equation by plotting ln k/T against 1/T (Table 4) :
ln k/T = ln K/h + ∆S≠/R – ∆H≠/RT
Where; k is the rate constant, T is absolute temperature, K is Botteman constant, h is plank constant and R is universal gas constant.
Table 3 : Effect of temperature on bromination of o-cresol by N-bromopthalimide.
| Temperature | 1/T /10-3K-1 | Specific reaction rate, K/ M-1 S-1 | Log K | |
| t/0C | T/K | |||
| 20 | 293 | 3.41 | 140 | 2.15 |
| 25 | 298 | 3.35 | 175 | 2.24 |
| 30 | 303 | 3.30 | 240 | 2.38 |
| 35 | 308 | 3.25 | 309 | 2.49 |
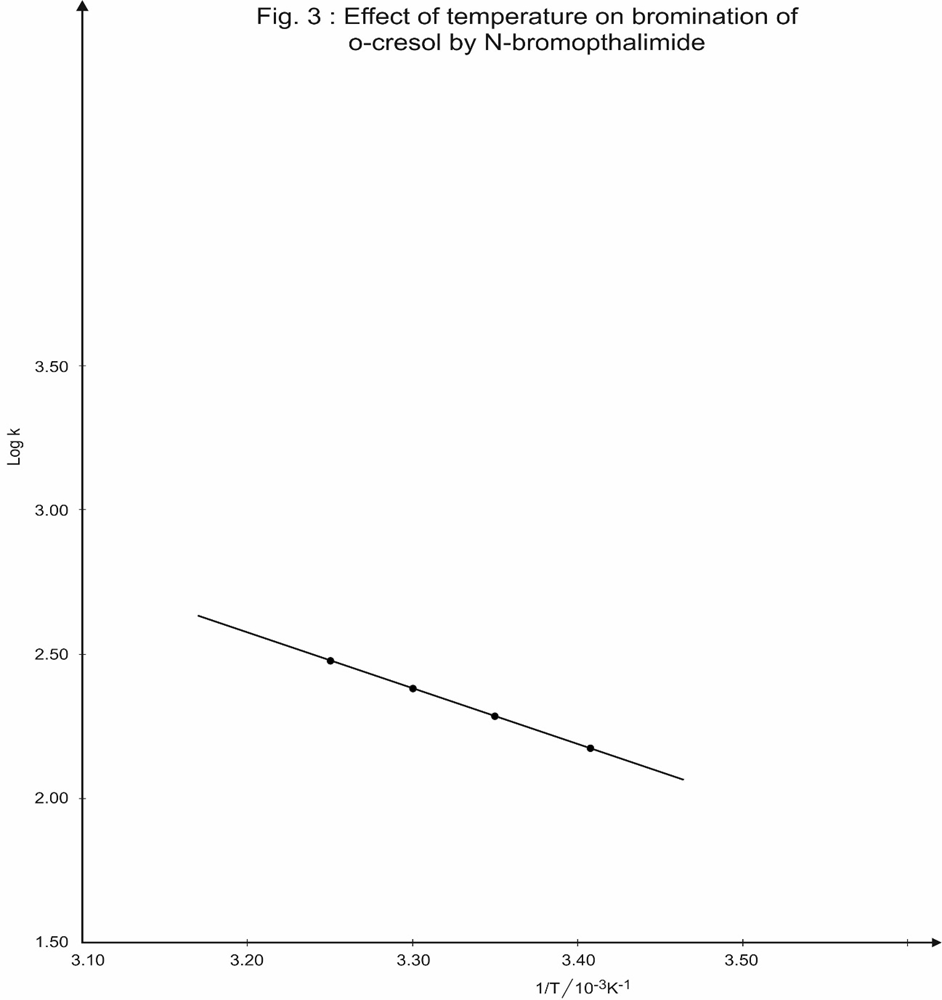
Slope of the plot of log K versus 1/T = – 2.125 x 10-3
\Energy of activation, Ea = 40.7 kJ M-1
In N-Bromopthalimide, N-atom posses more electronegativity than the Br-atom. Hence Nitrogen and Bromine atom carry negative and positive charge respectively. Therfore, it may possible that N-Bromopthalimide may complex with o-cresol, m-cresol and p-cresol in rate determining step to give intermediate complex, which decomposes by bromine transfer and in fast step proton will be abstracted by N-Bromopthalimide anion to give Bromocresol and pthalimide as product. From observed kinetic and thermodynamic parameters the most probable mechanism proposed for o-cresol (as typical case) is as below;

Mechanism of bromination of o-cresol
In case of m-cresol, para product is obtained while ortho product is obtained for p-cresol. In ortho and para isomers of cresol the ortho and para directing effect of -OH and -CH3 group is not in unison Hence the reactivity of these two isomers is lower. m-cresol shaws higher reactivity due to unison effect of -OH and -CH3 group. There is steric hindrance of -OH and -CH3 in o-cresol and hence shows lower reactivity among all three isomers. Therefore the reactivity of these isomers are as below,
m-cresol > p-cresol > o-cresol
Table 4 : Kinetic and thermodynamic parameters of kinetics of bremination of regiosomers of eresol by N-bromopthalimide in aqueous solution at 250C
| Parameters | O-cresol | m-cresol | p-cresol |
| Specific reaction rate, K/M-1S-1 | 175 | 7398 | 410 |
| Energy of activation Ea/KJ mole-1 | 40.7 | 21.7 | 34.5 |
| Frequency factor A / M-1S-1 | 2.37 x 109 | 4.70 x 107 | 4.56 x 108 |
| Entropy of activation DS# / KJ mole-1 | -73.76 | -106.34 | -87.48 |
CONCLUSION
The rate constant values obtained in this study for the bromination of regioisomers of cresol endorse the relative reactivity of these isomers and in accordance with the steric compulsions that prevails in these reactions. The rate constants of bromination of the regioisomers of cresol studied are seen to follow the following order.
o-cresol > p-cresol > m-cresol
Amongs these isomers, o-cresol has relatively slowest rate of bromination than p-cresol and m-cresol. The increasing values of rate constant are seen accompany decreasing frequency factors, increasing activation energies and more negative values of entropies of activation. (Table 4). These trends are indication of the reactivity order of isomeers of cresol in quantitative manner from observed kinetic and thermodynamic data.
ACKNOWLEDGEMENT
Author is thankful to Institute, for necessary facilities to carry out present investigation.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this paper.
REFERENCES
- Pereira S.S.; Oliveira H.M.; Turrinin R.N.; Lacerda R.A.; Disinfection with sodium hypochlorite in hospital environmental surfaces in the reduction of contamination and infection prevention : Asystematic review. Rev Esc Enferm Us., 2015, 49(4), 681-688.
- Mc Donnell G.; Russell A.D.; Antiseptic and disinfectants : activity, action and resistance, clin. Microbiol. Rev., 1999, 12(1), 147-179.
- Castelo – Branco P.A.; Rubinger M.M.M.; De C Alves L et sl-synthesis and antifungal activity of halogenated aromatic bis-r-lactones analogous to avenaciolide. Quim Nova Sao Paulo. 2012, 35(5), 948-955.
- Totter F.; Rittmeyer P.; Organometallics in synthesis. Chichester: Wiley; 1994, 167-194.
- Bodineau N.; Mattalia J.M.; Thimokhin V.; Handoo K.; Negrel J.C.; Chanon M.; Formation of Grignard reagent from aryl halides : effective radical probes hint at a nonparticipation of dianions in the mechanism. Org. Lett; 2000, 2(15), 2303-2306.
- Najera, C.; Sansano, J.M.; Yus, M.; Recent synthetic uses of functionalized aromatic and heteroaromatic organolithium reagents prepared by non-deprotonating methods; Tetrahedron, 2003, 59(47), 9255-9303.
- Canon, K.C.; Krow, G.R.; Handbook of Grignard Reagents, Dekker, New york, 1996.
- Dangat V.T.; Bonde S.L.; Borkar V.T.; and Maske P.D.; Rapid kinetics of chlorination of Thiophene in aqueous medium using Rotating platinum electrode. Research Journal of chemical sciences. 2012, 2(7), 75-79.
- Bonde S.L.; Dangat V.T.; Borkar V.T. and Yadav R.P.; Rapid Iodination of Xylidines in Aqueous medium: Kinetic verification of speculated Reactivities. Res. J. Chem. Sci., 2012, 2(6), 1-5.
- D. B. Patil; S.B. Kapoor; Effect of bromide ion on the kinetics of bromination of o-hydroxy benzoic acid by bromine in aqueous solution. Arabian Journal of chemistry. 2013, 6, 353-360.
- Kolthaff I.M.; and Laitinen H.A.; Voltammetric determination and Amperometric Titration with a rotating microelectode of platinum wire. Journal of physical chemistry. 1941, 45(7), 1079-1093.
- Borkar V.T.; and Dangar V.T.; Structure – Reactivity correlation of Thiophene and Thiphene – 2 – Sulponic Acid by Investigation of Rapid kinetics of Bromination in aqueous medium. Research Journal of chemical sciences, 2014, 4(12), 48-51.
- Bhore J.B.; Shubham Nimbalkar; Gatkul B.I.; Shinde M.P.; The kinetic study for the Fast Bromination Reaction of the Regioisomers of cresol in Aqueous medium by competition Techniques. IOSR Journal of Applied chemistry, 2019, 12(5), 13-18.
- S. Mohamed Farook; S. Shivakamasundari and S. Viswanathan; Kinetics of chlorination of certain aromatic compounds using N-chlorosuccinimide. Indian Journal of chemistry, 1984, 23 A, 239-240.
- Sandya B. Walke; Ranjana P. Bhadane; Sulbha B. Deokar; Shantaram L. Bonde; Kinetics of Rapid Chlorination of Imidazole by N-chlorosuccinimide in Aqueous medium and study of Antimicrobial Activity of its chloro Derivative. International Journal of chemistry and pharmaceutical sciences, 2016, 4(2), 61-69.
- Vivek Sharma; Priyanka Srivastava; Santosh Kumar Bhardwaj; and D.D. Agrawal; Green process synth, 2018, 7, 477-486.
- Radhakrishnamurti P.S.; and Sahu S.N.; Kinetics of Nuclear Bromination of Aromatic Amines by N-Bromosuccinimide. Indian Journal of Chemistry, 1977, 15 A, 785-787.
- Rao T.S.; and Dalve S.P.; Kinetics and mechanism of the bromination of aromatic substrates by N-Bromosuccinimide in aqueous solution, current science, 1984, 53(5), 258-259.
- Chafle D.M.; and Anande H.D.; voltammetric kinetics of bromination of the regioisomers of Nitrophenol by N-Bromosuccinimide in aqueous solution; IOSK Journal of Applied Chemistry, 2023, 16(6), 49-58.
- Chafle D.M.; and Anande H.D.; voltammetric kinetics of bromination of regioisomers of chlorophenol by N-Bromosuccinimide in aqueous solution, International Journal of creative Research Thoughts, 2023, 11(6), 795-801.
- Birla Anjaich; Kethavath Prameela; Pabba Srinivas; Kamatala Chinna Rajanna; Int. J. Chem-kinet; 2018, DOI : 10.1002 / Kin. 21214, 1-9.
