Targeted drug delivery (TDD) is intended to establish the medication in a certain tissues of interest, with reduced concentration on other tissues. Wherefore, the drug is targeted to its designated site. Therefore, the drug does not have any adverse effects on the surrounding tissues. Furthermore, the drug remains localized, preventing any loss, thereby ensuring maximum efficacy of the medication [1]. The various vehicles, such as synthetic polymers, immunoglobulin, serum proteins, liposomes, niosomes, microspheres, and erythrocytes, have been employed for targeted drug delivery. Amongst, Niosomes are considered to be the improved vehicle to enhance bioavailability and therapeutic efficiency by acting as a trusted approach for disease treatment and minimizing the side effects [2, 3].
In recent years, research efforts have concentrated on the development of alternative drug delivery systems, with the goal of overcoming the constraints of traditional dosage forms and ensuring enhanced bioavailability, fewer side effects, controlled drug release, and targeted administration. Niosomes demonstrate pharmacologically acceptable characteristics, including potential for enhanced drug bioavailability, stability, sustained action, biodegradability, alteration of drug distribution and non-toxicity [2-4]. Niosomes are nano-sized layered structures ranging from 10 to 1000 nm in size. The central component of these structures is composed of substances that are both environmentally friendly and non-reactive towards the human immune system. Additionally, biocompatible surfactants are utilized in the formation of niosomes.
Polymersomes, liposomes, polymer-based vesicles, micelles, and niosomes are examples of nano-carriers that can be used to transport therapeutic drugs to disease-specific locations [4]. Nanocarriers have garnered significant attention from researchers due to their numerous advantages, including the capacity to prolong the serum half-life of medications, impede absorption by reticulo-endothelial systems (RESs), and minimizing non-specific adsorption by optimizing their constituents or generating a multifunctional surface. It can be safeguard the medicine during in vivo circulation and storage [1-5]. Nano vesicles are often utilised as vehicle for chemical pharmaceuticals, protein therapeutics, and gene therapies. Niosomes exhibit a higher degree of stability in comparison to lipids due to the inherent stability of their constituent elements, namely non-ionic surfactants, which possess superior chemical and physical stability characteristics [5]. Improved stability of the niosomes made formulation processing considerably easier. Furthermore, niosomes are substantially less expensive than liposomes. According to these advancements and the benefits of niosomes, this study introduces the structure, components, and formulation processes, as well as their prospective therapeutic uses [6-8].
2. THE STRUCTURE AND COMPOSITION OF NIOSOME
2.1. The Structure of the Niosome:
The identification of the fundamental structural constituents of niosomes is of paramount importance as it has a significant impact on the selection of chemicals that can be employed in the formation of niosomes, as well as the packing mechanism of pharmaceuticals for delivery. Niosomes, akin to liposomes, are vesicles composed of non-ionic surfactants that exhibit a bilayer structure (Fig. 1). Aqueous fluids have polar heads, while organic solutions have hydrophobic heads [9]. Bilayer vesicles are classified as unilamellar or multilamellar (Fig. 2) [8-10]. Multilamellar vesicles (Fig. 2, 2.a, 2.b and 2.c) are concentric rings formed by at least two bilayer vesicles or a big vesicle containing more than one tiny carrier. As a result of this, multilamellar vesicles often have greater particle sizes than unilamellar vesicles. Niosomes are typically sub-micron (colloidal) in size. Niosomes, which resemble liposomes, are stable colloidal particles prepared through the self-assembly of non-ionic surfactants and a hydrating mixture of cholesterol in an aqueous environment.
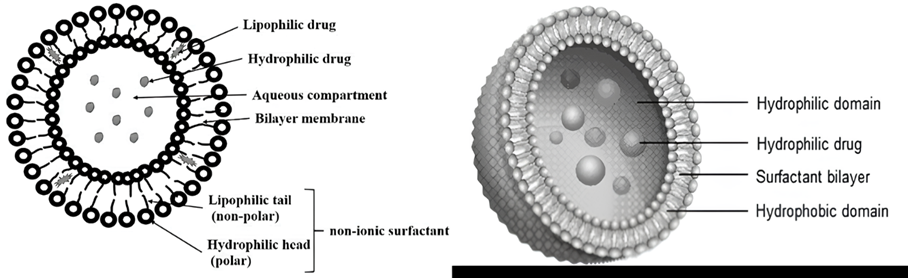
Figure. 1: The schematic representation on niosome structure [11, 12]
The small unilamellar vesicles (SUVs) exhibited particle dimensions ranging from 10 to 100 nm, while the large unilamellar vesicles (LUVs) displayed particle sizes within the range of 100 to 3000 nm. On the other hand, the multi-lamellar vesicles (MLVs) possessed particle sizes exceeding 5 µm, with a limited number of “giant” vesicles measuring greater than 15 µm being documented [11]. The schematic representation of niosome vesicular on their sizes as follows:
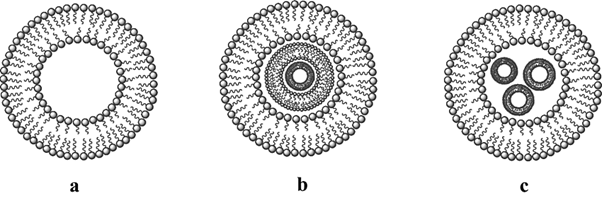
Figure. 2: The schematic representation on niosome vesicular size: 2.a Unilamellar vesicles (ULVs) and 2.b, 2.c the multilamellar vesicles (MLVs) [13]
Hydrophilic head group Hydrophobic side chain.
2.1.1. Small Lamellar Vesicles (SUVs):Small unilamellar vesicles (SUVs) are vesicles that have a single membrane layer and a diameter of about 20 to 100 nanometers. They are typically formed by sonication or extrusion of a lipid solution. Niosomes are vesicles that are made from non-ionic surfactants instead of lipids [9-11]. SUVs niosomes are a type of niosome that has a small unilamellar structure.
2.1.2. Large Lamellar Vesicles (LUVs): Large unilamellar vesicles (LUVs) are vesicles that have a single membrane layer and a diameter of about 100 to 1000 nanometers. They are typically formed by sonication or extrusion of a lipid solution. Niosomes are vesicles that are made from non-ionic surfactants instead of lipids.
LUV niosomes are a type of niosome that has a large unilamellar structure. LUV niosomes have several advantages over other types of niosomes. They are more stable and have a higher drug loading capacity [12]. They are also less likely to aggregate and can be easily formulated into different dosage forms.
2.1.3. Multilamellar Vesicles (MLVs): The structure comprises multiple bilayers that enclose the aqueous compartment individually. The vesicles exhibit diameters ranging from 0.05 to 10 µm. Among various types of niosomes, multilamellar vesicles have garnered significant attention in research. These vesicles are relatively easy to fabricate and possess remarkable mechanical stability, enabling prolonged storage durations [10-12]. These vesicles are greatly suitable for drug vehicle for hydrophobic compounds.
Table. 1: The niosome diameter of various sizes with their types
| Sr. No. | Types of Niosome | Size diameter of Niosome |
| 01. | Small Unilamellar Vesicles (SUVs) | Range = 0.025-0.05 μm (25-50 nm) |
| 02. | Multilamellar Vesicles (MLVs) | Range = less than 0.05 μm (50 nm) |
| 03. | Large Unilamellar Vesicles (LUVs) | Range = less than 0.10 μm (100 nm) [11-13] |
2.2.The composition of the Niosome:
Niosome is composed of cholesterol or its imitative, non-ionic surfactants, and occasionally, ionic amphiphiles with desired drug. Unlike liposomes, which are primarily composed of phospholipids, non-ionic surfactants serve as the primary constituent of niosomes. Niosomes have the capability to encapsulate drugs that possess both hydrophilic and lipophilic properties. Hydrophilic medications are accommodated within the core of the niosomes, while lipophilic pharmaceuticals are confined within the lipophilic region of the bilayer. To achieve the utmost stability in the formulation, an optimal amount of cholesterol is incorporated into the niosomes, owing to its interaction with non-ionic surfactants [13]. Cholesterol can only produce the bilayer structure, but it may get mixed in the bilayer membrane, managing the shape and flexibility of the membrane as a reliable buffers.
Amphipathic non-ionic surfactants that are used for niosomes include polysorbates [14], Spans [15], alkyl oxyethylenes [16] (often from C12 to C18), and others. Additionally cholesterol as membrane stabilizer. The utilization of the niosomal vehicle (consisting of Tween 60, Span 60, and cholesterol) has been found to substantially enhance the entrapment efficiency of drugs. This improvement can be attributed to the interaction occurring between the the acyl chains of Span 60 and drugs, as supported by previous studies [13-15].
In addition, certain charged molecule, namely phosphatidic acid and dicetyl phosphate (DCP) (negatively charged molecules), cetylpyridinium chloride and stearylamine (SA) (positively charged molecules), are employed in niosomes are three particular objectives: drug encapsulation, efficacy augmentation, and ICH based strength improvement [12-16].
Niosomes are a type of vesicular drug delivery system composed of non-ionic surfactant. Liposomes are structurally similar, made from amphiphilic non-ionic surfactants in place of phospholipids. Niosomes can encapsulate both hydrophilic and lipophilic pharmaceuticals, giving them an adaptable drug delivery system. There are mainly four components (Fig. 3) are used for the preparation of niosome:
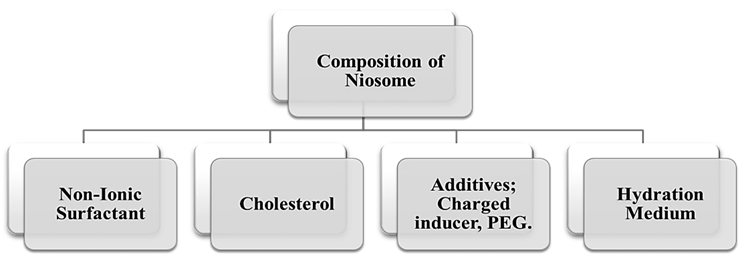
Figure. 3: The structural composition of Niosome formulation
2.1.1. Non-Ionic Surfactant: Surfactants are essential in the preparation of niosomes. Non-ionic surfactants such as Tweens (tween20,40,60,80), Spans (span60,40,20,60,85,80), and Brij (Brij 30,35,52,58,72,76) are usually utilised in the formation of niosomes. The hydrophilic head of the non-ionic surfactants is followed by a lipophilic tail. The selection of a non-ionic surfactant is illustrated by the hydrophilic and lipophilic balance (HLB), critical packing of amphiphiles and critical micellar concentration (CMC) [14-16]. The non-ionic surfactant employed to produce niosomes has the potential to influence their size, stability, and drug loading capability. Non-ionic surfactants with longer alkyl chains tend to form larger niosomes with higher drug loading capacity [17].
Table. 2: The list of non-ionic surfactant according to their hydrophilic and lipophilic nature of drug medicament
| Nature of Non-Ionic Surfactant | Types of Non-Ionic Surfactant | Drug Choice in niosome formulation |
| Lipophilic HLB (4-8) | Sorbitan monolaurateSorbitan monopalmitateSorbitan stearateSorbitan trioleate | Hydrophilic drugs, ex: ibuprofen [18], naproxen [19], ACE inhibitors [20], and Antihistamine [21]. |
| Hydrophilic HLB (8-14) | Polyoxyethylene (20) sorbitan monolaurate Polyoxyethylene (20) sorbitan monopalmitate Polyoxyethylene (20) sorbitan stearate Polyoxyethylene (20) sorbitan monooleate | Lipophilic drug, ex: acyclovir, paclitaxel, doxorubicin and piroxicam, meloxicam [22-26] etc. |
| Amphiphilic HLB (9.7-18) | Brij (polyoxyethylene (20) alkyl ether) 30,35,52,58,72,76.Lauryl (C12), cetyl (C16), Stearyl (C18), oleyl (C18:1), lauryl (C12), and cetyl (C16) respectively. | Both Hydrophilic and Lipophilic drug ex: tetracycline and erythromycin and itraconazole [27-29] etc. |
2.1.2. Cholesterol: Cholesterol is one of the most critical components of noisome. In the bilayer structure, it creates a hydrogen bond with the surfactant’s hydrophilic head. It can affect several crucial carriers with features including entrapment efficiency (%EE) as well as improve stability. It also improves bilayer surface stability by changing the gel liquid transition temperature. If surfactants, HLB > 6 is required to form bilayer vesicles, and adding cholesterol improves stability at lower HLB values [38]. Its composition also influences loading capacity, which is a crucial aspect in niosomal formulation. It has also been demonstrated that in the case of more lipophilic surfactants, the inclusion of cholesterol aids in the inhibition of aggregation and the creation of vesicles [13-15]. To increase niosome stability and drug loading capability, cholesterol can be added. Cholesterol, on the other hand, can cause niosomes to grow in size.
2.1.3. Additives:
- Charge Inducers: The primary goal of adding charged groups to the bilayer surface is to enhance stability. Dicetyl phosphate is primarily utilised as a charged molecule that imparts negatively charged molecules on the bilayer surface. It is primarily used to promote vesicle stability and avoid aggregation by supplying charged groups over the bilayer surface. The Dicetyl phosphate is a charged chemical that adds a negative charge to the bilayer surface. Generally in the range of 2.5-5 mol.percentage. Enhance the quantity of charge molecule, however, inhibits niosome production [14-16]. The list of charge inducer depending on their charges as below followings in Table. 3:
Table. 3: The list of charge inducers in niosome formulation
| Charge Inducers in Niosome Formulation | ||
| Positive (+ Ve) | Stearyl Amine (SA) | Stearyl Pyridinium Chloride [40] |
| Negative (- Ve) | Dicetyl Phosphate (DCP) | Phosphotidic acid [15, 16] |
- Cationic/Helper Lipids Elaboration of Niosomes: Niosomes are vesicular systems that are similar to liposomes in structure and function. They can be used to encapsulate a variety of drugs and other bioactive agents, including genes. Cationic/helper lipids are a type of lipid that can be used to enhance the gene delivery efficiency of niosomes. Cationic/helper lipids work by interacting with the negatively charged cell membrane and promoting the re-uptake of the niosomes into the cell [14]. There are a number of different cationic/helper lipids that can be used to elaborate niosomes as a gene delivery platform. Some of the most common cationic/helper lipids include:
- Dioctadecyldimethylammonium bromide (DOTMA)
- Dioleoylphosphatidylethanolamine-N-[3-(2-hydroxyethyl)proprionamide] (DOPE)
- Stearyltrimethylammonium bromide (STAB
- Cholesterol
Cationic/helper lipids can be incorporated into niosomes using a variety of methods, including the melt method, micelle solution method, and enzymatic method. The improved method to use will based on the type of cationic/helper lipid being used and the desired properties of the niosomes.
- Polyethylene Glycol (PEG): Polyethylene glycol (PEG) is a non-ionic polymer that can be used to modify the surface of niosome. PEGylation of niosomes has a number of advantages, including:
- Increased circulation time in the bloodstream: PEGylation can reduce the uptake of niosomes by the reticuloendothelial system (RES), i.e., is a network of organs and tissues that removes foreign particles from the bloodstream. This can increase the circulation time of niosomes in the bloodstream and allow them to reach their target tissues.
- Reduced immunogenicity and Increased stability [13-15].
PEG can be incorporated into niosomes using a variety of methods, including the melt method, micelle solution method, and enzymatic method.
- Hydration Medium: In the preparation of niosome, the hydration medium, also known as the aqueous phase, is a crucial component that plays a significant key role in the formation of niosomal vesicles. The hydration medium typically consists of water or an aqueous buffer solution. The choice of hydration medium can influence the characteristics of the resulting niosomes, including size, stability, and drug encapsulation efficiency. For example, a more polar hydration medium will tend to form smaller niosomes with a higher drug loading capacity [15-17]. The hydration temperature is the temperature at which the niosomes are formed. It is important to control the hydration temperature because it can influence the size, drug loading capacity and stability for the niosomes. The various commonly used hydration media for niosomes include:
Table. 4: The list of some common hydration media used in niosome formulation
| Hydration medium | Advantages | Disadvantages |
| Water | Simple to use and cost-effective | Can lead to the formation of niosomes with a wide range of sizes |
| Buffered saline (PBS) | Maintains the pH of the niosomal dispersion, which can improve the stability of the niosome | Can increase the cost of the formulation |
| Phosphate buffer | Can be used to optimize the size and charge of the niosome | Can increase the complexity of the formulation process |
| Glycerol solution | Can be used to decrease the freezing point of the niosomal dispersion, which can improve the stability | Can increase the viscosity of the niosomal dispersion, which can make it difficult to administer [12-17] |
The each compound in the formulation of niosome having lots of merits in the niosome from initial to the formation and till their stability having different function. The common purpose of the composition of niosome formulation as shown in the give Table.5 below following discussion.
Table 5. The common objective of the composition of Niosome formulation
| Niosome Components | Specified objectives |
| Surfactants | The vesicle membrane and control its properties, such as size, stability, and drug loading capacity. |
| Cholesterol | Stabilizes the vesicle membrane and prevents it from aggregating. |
| Charged Molecule | To prevent the aggregation of molecules of vesicles. |
| Drugs | The API that is encapsulated in the niosomes. |
| Hydration Medium | Use to hydrate the vesicle formulation [7, 13, 14, 17]. |
These above the main objective of all excipients involving in the niosomal formulation as well as the preparation. The composition of the niosomal formulation is carefully designed to achieve the desired drug delivery profile. For example, the type of non-ionic surfactant as well as the quantity of cholesterol employed is going to alter the stability, rigidity, and drug loading capacity of the niosome. The aqueous phase may also be modified to improve the solubility of the drug or to enhance the targeting ability of the niosomes.
- THE FORMULATION DEVELOPMENT AND EVALUATIONS OF NIOSOME
The fabrication and preparation of niosome Nano vesicles can be accomplished through various methodologies, each of which presents distinct advantages. It is worth highlighting that the production process significantly influences the ultimate characteristics of niosomes. An overview of the various techniques for niosome preparation is given in this section. Depending of the size the formulation methods will be summarized in Table. 6:
Table. 6: The preparation method of niosome depending on their vesicular diameter
| Niosome Preparation methods | SUVs | LUVs | MLVs |
| Sonication, Ethanol/Ether Injection (EI), Homogenization | Extrusion | Thin film hydration (TFH) method | |
| REV | Reverse Phase Evaporation (REV) | Solvent injection | |
| Micro emulsion | Freeze-Drying (Lyophilization) and other |
- Formulation methods of Niosome:
- Formulation of Niosome by TFH, Hand Shaking Method:
Thin film hydration (TFH) is also known as hand shaking methods which is one of the most used methods for producing liposomes. This approach could also be used for producing niosomes. Initially, dissolve the non-ionic surfactant and cholesterol in such solvent chloroform or ethanol. The ratio of surfactant to cholesterol is typically 2:1, but this can be varied depending on the desired properties of the niosomes. Deposit the organic solution onto a round-bottom flask and rotate the flask under vacuum to evaporate the solvent. This will form a thin film of surfactant and cholesterol on the bottom of flask. As shown in the (Fig. 4) after, add an aqueous phase to the round-bottom flask and gently shake the flask by hand to hydrate the lipid film. The aqueous phase can be water or a buffered saline solution. Sonicate the round-bottom flask for a few minutes to form the niosomes [32].
Centrifuge the round-bottom flask to separate the niosomes from the aqueous phase. The niosomes can then be collected and used for further applications. Multilamellar vesicles (MLV) are niosomes generated using the TFH method. This process is commonly used to create niosome containing pharmaceuticals like aceclofenac niosome and pro-niosome [30, 31], diclofenac sodium [57], and naproxen [34, 35].

Figure. 4: The formulation of niosomes via TFH method [61].
- Ether Injection Method (EIM): Surfactant along with additives are soluble in diethyl ether (organic solvent), and subsequently extruded with care via injection into an aqueous solution. This aqueous solution, which holds the desired medicament, is assisted at a consistent temperature of approximately 60 °C. A rotary evaporator evaporates the organic solvent [8]. Surfactants play a pivotal aspect in the preparation of single-layered vesicles through the process of ether vaporization. The ether injection method yields SUVs and LUVs with a substantial encapsulated aqueous volume. The ultimate vesicle size might range from 50-1000 nm based on the conditions (Fig. 5).
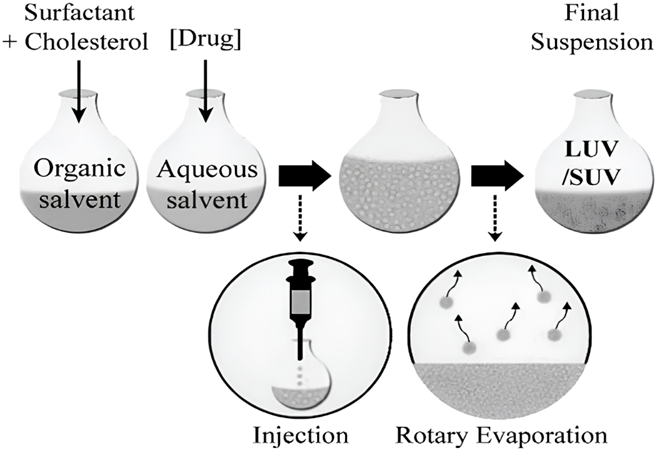
Figure. 5: The niosomal preparation via ether injection method [62]
This ether injection method (EMI) widely used in the formulation of Aspirin [36] and Nimesulide [37] etc.
- Solvent Injection (SI) Method: Diethyl ether and ethanol are utilized in the solvent-induced technique (Fig. 6) to dissolve surfactants and other additive. The resulting homogeneous solutions is subsequently loaded into a syringe pump and administered drop by drop through a needle into an aqueous solution containing the drug, which is maintained at a sustained temperature greater than the boiling point of the organic solvent. A rotating vacuum evaporator totally evaporates the residual organic solvent. Unilamellar vesicular niosomes of various sizes are generated during this evaporation process, and the encapsulated aqueous volume is substantially bigger than in previous approaches [39-40]. This approach is used to make elastic niosomes out of tween 80 and span 60 for the entrapment of the diclofenac sodium [57]. They employed ethanol as a solvent for span 60 and diclofenac sodium, and then injected the solution into an aqueous phase containing tween 80.
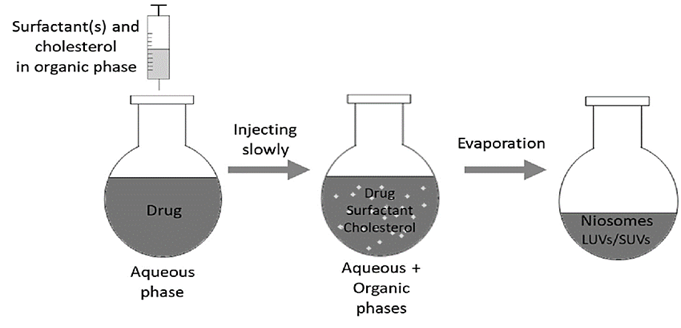
Figure. 6: The niosomal formulation via SI method [7]
- Transmembrane pH gradient method (Remote Loading Technique): If the pH of the niosome vesicles is higher on the outside, the basic medication passes through the membrane barrier. Because of the decreased pH inside the niosome, the basic medication becomes ionised and precipitates. As a result, after encapsulation, it is unable to leave the vesicle [42]. This process can be demonstrated experimentally by vortex mixing a thin coating of surfactant and cholesterol with acidic medium i.e. citric acid (pH 4.0). As a result, the MLVs (Fig. 7) are frozen and thawed. The drugs are suspended in an aqueous solution and vortex. The pH is then elevated to 7.0-7.2 before being kept at high temperature of 60 °C for 10 minutes to produce niosomes [43].
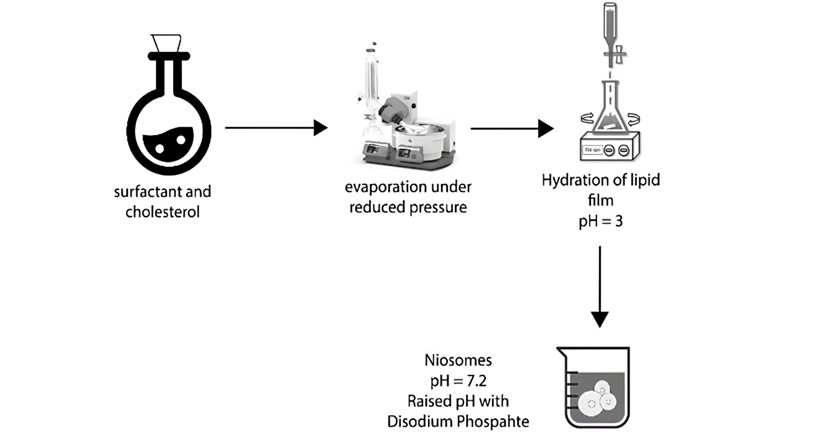
Figure. 7: The schematic representation of niosomal formulation via pH gradient method [3]
- Probe Sonication Method: A widely accepted technique for the formulation of niosomes, which is both user-friendly and straightforward. The procedure includes the incorporation of a drug solution (in buffer) to a carefully selected blend of non-ionic surfactants, at an optimized ratio, followed by sonication at a predetermined temperature, frequency, and duration to achieve the optimum niosomes. It is an effective method for controlling the particle diameter of the niosomes. Sonication has been shown to reduce the size of niosomes with a restricted size distribution [44-45]. However, probe sonication uses a high energy, which can result in a fast increase in temperature and titanium shedding.
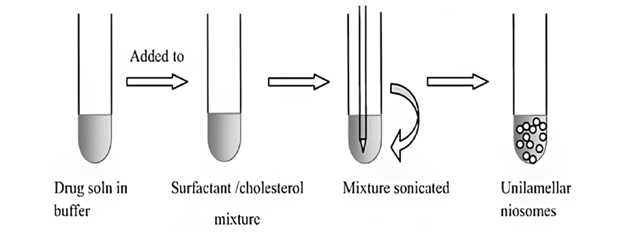
Figure. 8: The schematic representation of niosomal formulation via sonication [53]
- Pro Niosome or Dry Niosome: Proniosomes, alternatively referred to as dry niosomes, are dry formulations of non-ionic surfactant vesicles that can be rapidly converted into niosomes upon hydration. These formulations are increasingly employed in niosome formulation owing to their exceptional stability [6-9]. Proniosomes are non-ionic surfactant-coated water-soluble carriers that may be quickly hydrated into niosomes before usage (Fig. 9). This approach presents several benefits, encompassing enhanced physically and chemically stability for extended storage, convenient transport, and scalability [43]. Moreover, this technique holds the potential to expand the manufacturing possibilities of niosomes into other forms, like as gels and tablets. Detailed research has demonstrated that proniosomes have shown promising potential for effective medication administration through various routes, such as periodontal and transdermal routes [46-48].
This technique is highly efficient in decreasing the amount of water for niosomes, thereby enhancing their stability and offering a potential resolution for prolonged storage. It has been successfully employed in formulating Ketoprofen [43], Ketorolac [44], and Tenoxicam [45].
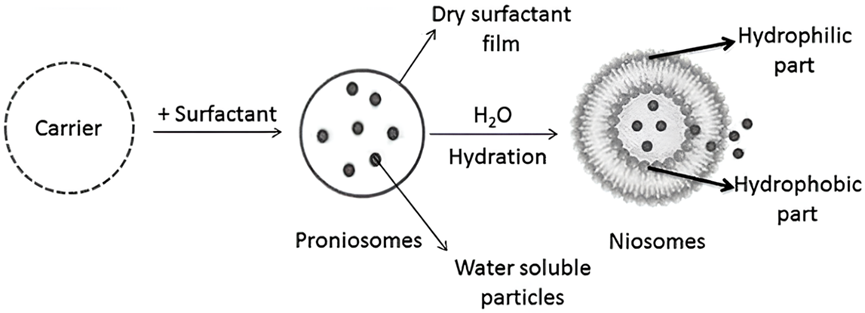
Figure. 9: The preparation of niosomes via pro-niosomes method [21]
- The Bubble Method: A novel technique has been recently developed to facilitate the production of niosomes, eliminating the need for organic solvents. The process involves employing a specialized apparatus comprising a spherical bottom flask with three necks that was immersed in a water bath to ensure precise temperature. The first and second necks include a water-cooled reflux and thermometer, while the third neck is used to provide nitrogen (Fig. 10). At a temperature of 70°C, a mixture of cholesterol and surfactant is uniformly distributed in a buffer solution with a pH of 7.4. Dispersion is subjected to a blending process at a duration of 15 seconds using a high shear homogenizer. Subsequently, the mixture is exposed to nitrogen gas at 70°C, resulting in the formation of niosomes [49, 50].
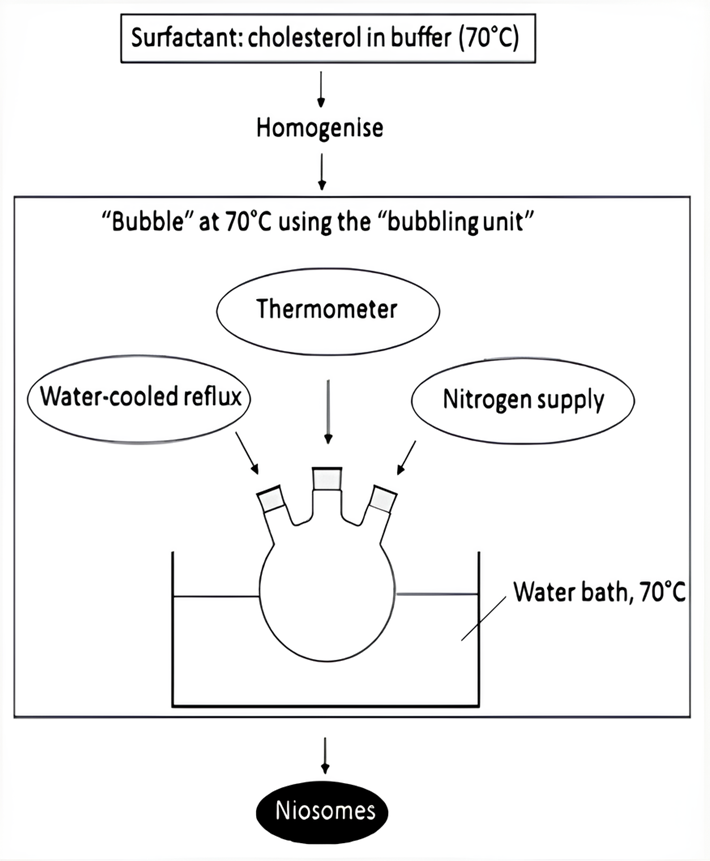
Figure. 10: The bubble method in formulation of niosomal preparation [53]
- Heating Method (HM): The various non-ionic surfactants and certain additives, such as cholesterol, hydrate with individually for one hour at room temperature in a nitrogen environment in PBS (pH = 7.4). The solution is then heated on a hot-plate stirrer (about 120 °C) for around 15-20 minutes to dissolve cholesterol. Following the reduction of temperature to 60 °C, the supplementary constituents, namely surfactants and other additives, are introduced into the buffer solution that already contains the dissolved cholesterol. Subsequently, the mixture is subjected to continuous stirring for an additional duration of 15 minutes [51]. Niosomes produced at this stage are kept for 30 minutes at 4-5 °C in a nitrogen surrounding before it is needed [51-52].
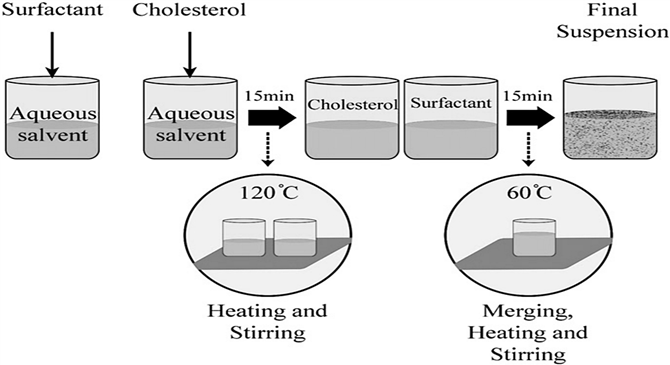
Figure. 11: The systematic representation for niosome formulation through HM [62]
- Reverse Phase evaporation (REV) Method: As illustrated in Fig. 12, the REV technique necessitates the dissolution of the non-ionic surfactant and cholestrol to an organic solvent. Prior to being subjected to sonication with the organic phase to generate an emulsion, the loaded medication is solubilized to an aqueous solution, like PBS or water. The organic solvent is evaporated using a rotating vacuum evaporator with temperature of 40-60 °C for the formation of niosomes. In contrast to the TFH technique, the REV method demonstrated the production of nanoparticles characterized by consistent size and unilamellar or oligolamellar structures. This REV process was employed in the development of diverse niosomal preparations for analgesic drugs, such as acetazolamide [51].
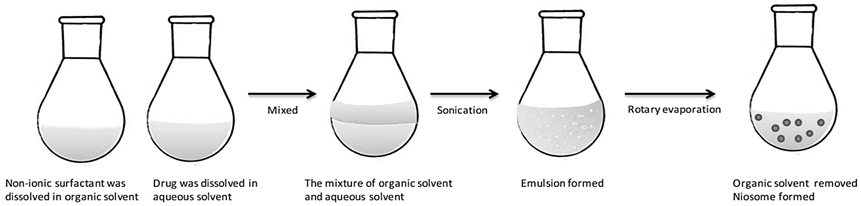
Figure. 12: The formulation of niosomes Via REV method [61]
- Micro Fluidization Method: Dissolve the non-ionic surfactant with cholesterol to ethanol or isopropanol (organic solvent). Ratio for surfactant to cholesterol is typically 2:1, but this can be varied depending on the desired properties of the niosomes. Prepare an aqueous phase comprising of API or other molecule to be encapsulated. The aqueous phase can also contain other components, such as buffers, salts, and excipients. Load the organic and aqueous phases into the microfluidic device. Apply high pressure to the microfluidic device to mix the two phases. This will result in the preparation of niosome. Collect the niosomes from the outlet of the microfluidic device. The microfluidization method is a powerful tool for the formulation of niosomes [52].
This technique holds the potential to generate niosomes possessing a diverse range of desired characteristics, including size, encapsulation efficiency, and release profile. Consequently, this approach is regarded as a favourable avenue for the production based advancement of niosomes.
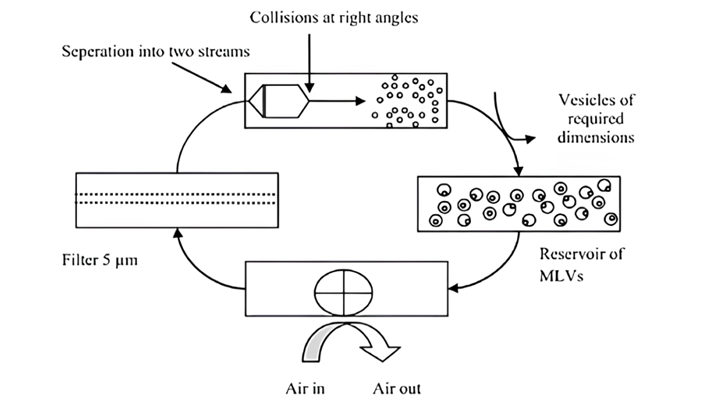
Figure. 13: The schematic representation of niosomal formulation via micro-fluidization method [53]
- Others: Melt Method, the simplest and most commonly used method for preparing niosomes. It involves melting the surfactant and cholesterol at a temperature above their melting points. The aqueous phase is then added and the mixture is sonicated to form a dispersion of niosomes [53]. Micelle solution and enzymatic method, it involves dissolving surfactant and cholesterol in ethanol. Subsequently, the water soluble phase is introduced and the amalgamation is agitated to generate a micellar solution. The micellar solution is subsequently subjected to sonication, resulting in the formation of a dispersion comprising niosomes. Enzymatic method, similar to the micelle solution method, but the micellar solution is treated with an enzyme such as phospholipase D to form a dispersion of niosomes [54].
- Characteristics and evaluations of niosome
Typically, the evaluation of niosomes encompasses an examination of their size distribution, surface morphology, drug loading efficiency, stability and zeta potential throughout the formulation as well as storage. These attributes hold significant importance for niosomes as they not only impact the stability, entrapment rate of the niosomes, in fact it also influence their in vivo performance [52-54]. As the field of detection technology continues to progress, an increasing number of methodologies are being devised to measure niosomes. Table G provides a comprehensive overview of commonly employed techniques for the characteristics of niosomes.
Table.7: The characteristics and evaluation of niosome
| Characterization | Evaluation parameters |
| Vesicular Size diameter and Size distribution | CLS, SEM, STM, AFM, DLS |
| Zeta potential | DLS, mobility |
| Entrapment efficiency (%EE) | Entrapment Efficiency (%EE) = HPLC, UV/VIS, and fluorescence are used to measure the quantity of the loaded medication. |
| Stability | DLS was employed to determine size and zeta potential under in vivo conditions (370 C or serum), while drug leakage was loaded. |
DLS (Diameter laser scatter), SEM (scanning electron microscope), AFM (Atomic Force Microscope), STM (Scanning Tunnelling Microscope), and HPLC (High Performance Liquid Chromatography) are other abbreviations.
- Vesicular Size diameter and Size distribution of Niosome: Niosomes are spherical in form, and their size may be evaluated using a variety of methods, as summarised in Table 10. Typically, their size distribution and polydispersity index are calculated. Niosomes exhibit a spherical morphology, and their dimensions can be determined through various techniques, as outlined in Table 2. Laser scattering (DLS) particle size analyzers are commonly used to measure their size distribution and polydispersity index. TEM, SEM, AFM, and STC are utilised to regulate the morphology of the niosomes in order to better detect their sharpness. SEM and TEM pictures were used to examine the shape of blank niosomes and three types of medicines, Etodolac [55], diclofenac diethyl ammonium [56], diclofenac sodium [57], and aspirin-loaded niosomes [58].
The assembly of niosomal formulations may be influenced by various factors, including the structural characteristics of non-ionic surfactants, which can significantly impact the size, shape, and stability of niosomes. 1) The HLB of the surfactant is a key factor, as surfactants with a higher HLB tend to form smaller and more stable niosome. 2) Membrane additives is cholesterol used to improve the stability and rigidity of niosomes. 3) Type of encapsulated drug, can also affect the assembly of niosome. Lipophilic drug may tend to interact with the lipid bilayer, while water loving drug may remain in the aqueous core. This can affect the size, shape, and stability of the niosome. 4) Surfactant and lipid levels, in the niosome formulation is another important factor. A higher concentration of surfactant will generally result in the formation of smaller niosomes, while a higher concentration of lipid will increase the stability of the niosome. 5) Hydration temperature, most non-ionic surfactants, the optimal hydration temperature is above the gel-to-liquid phase transition temperature. The diameters of the niosomes have been observed to range from roughly 20 nm to 50 m [60-63]. The zeta potential of niosomes in solution is critical for their stability in solution and may be evaluated using Zetasizer, microelectrophoresis, and DLS equipment. The SEM/TEM of some analgesic drug shown in the (Fig. 14) and their diameters mentioned in the Table. 10.
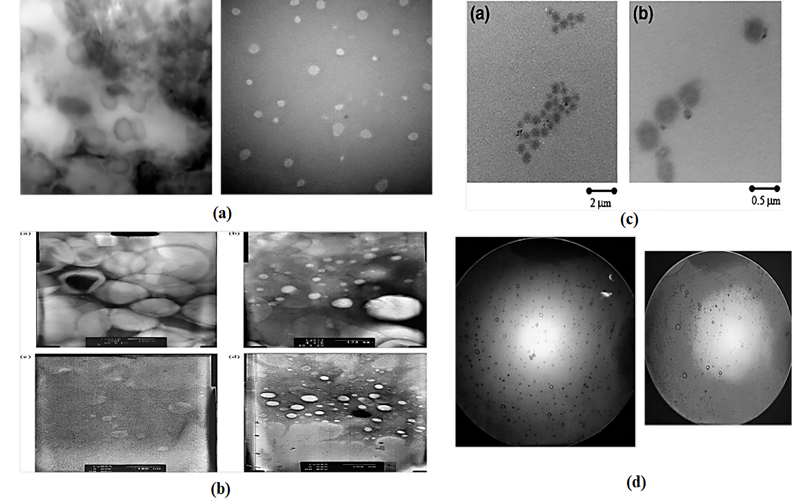
Figure. 14: The schematic representation of (a) The TEM view of Etodolac [55],(b) The TEM view of diclofenac diethylammonium [56],(c) The TEM view of Diclofenac Na Gel [57] and (d) The optical microscopic view of Aspirin Optical View [36] respectively
- Zeta Potential of Niosomes: Zeta potential is a crucial parameter in niosomal characterization. It measures the surface charge of the niosomes. A high zeta potential (greater than +30 mV) indicates that the niosomes are stable and will not aggregate. A low zeta potential (less than -30 mV) indicates that the niosomes are unstable and may aggregate. Zeta potential is affected by a number of factors, including the type of surfactant used, the ratio of surfactant to cholesterol, the pH of the aqueous phase, and the presence of electrolytes. The optimal zeta potential may vary depending on the intended application of the niosomes [56-58]. As depicted in Table 8, the zeta potential of analgesic niosomal formulations can be evaluated through a range of techniques, including Zetasizer, mastersizers, microelectrophoresis, pH-sensitive fluorophores, high performance capillary electrophoresis, and DLS devices. These methods are effective in assessing the surface zeta potential of the formulations. The zeta potential of Lornoxicam [60], Naproxen [34] and Diclofenac sodium [57].
- PDI of Niosome: The polydispersity index (PDI) serves as a quantitative indicator of the size distribution within a particle population. PDI value is determined as the standard deviation of the particle size distribution divided by the mean particle size. A PDI value with 0 signifies, population where every particles are of identical size, whereas a PDI value of 1 signifies a highly heterogeneous population in terms of particle size. According to Table 8, Dynamic Light Scattering (DLS) particle size analyzers is applicable for evaluating the size distribution and polydispersity index (PDI) of niosomes. The PDI values for Meloxicam [59] and Lornoxicam [60] are provided in Table. 8 in a comprehensive manner.
Table. 8: The list of zeta potential and PDI of analgesic medicament in the niosomal formulations
| Drug Name | Zeta Potential | PDI (Polydispersity Index) | Ref. |
| Meloxicam | — | 0.15- 0.25 | [59] |
| Lornoxicam | -69 ± 7.4 mV | 0.492 – 0.259 | [60] |
| Naproxen | -31.9 mV | — | [34] |
| Diclofenac sodium | −49.4 mv | — | [57] |
- Entrapment Efficiency (%EE) of Niosomes: The ability of vesicles to effectively incorporate medicinal substances is quantified by the encapsulation efficiency of niosomes. The definition of niosome encapsulation efficiency is provided in Table 10 where the term “total weighed quantity” which refers to the amount of pharmaceuticals utilized in the preparation. The efficacy of niosomes in encapsulating active ingredients is primarily influenced by the type of non-ionic surfactant employed, the production methodology, and other components incorporated during the formulation process, including cholesterol. Studies have demonstrated that the entrapment efficiency can attain levels of 75%-90%, albeit typically falling within the range of 10%-40% [61]. The entrapment efficacy (%EE) of the some niosomal preparation including aceclofenac niosome [30] and aceclofenac proniosome [31], Nimesulide [37], naproxen [34], Etodolac [55] and diclofenac sodium [57] as analgesic niosomal formulation.
- Stability of Niosomes: The stability of niosome is a crucial factor in their preparation, which is influenced by various factors such as the preparation process, loaded medications, and the type of membrane-forming materials used. In order to evaluate the stability of niosomes during storage, it is imperative to assess changes in particle size, zeta potential, shape, and the rate of loaded drug leakage. In accordance with the ICH Guidelines for stability, as outlined in Table 9, an evaluation of niosome stability during circulation was conducted. The study considered three primary storage conditions, namely general storage, refrigeration, and freezer storage, to assess the stability of the niosomal formulation [37, 62].
Table.9: The storage condition for the Niosomal formulation as per the general case, refrigerator and freezer stability condition
| Case of Stability parameter | Study | Storing climate | Least duration for data submission time |
| General Case | Long term* | 25°C ± 2°C/60% RH ± 5% RH or 30°C ± 2°C/65% RH ± 5% RH | 12 months |
| Intermediate** | 30°C ± 2°C/65% RH ± 5% RH | 6 months | |
| Accelerated | 40°C ± 2°C/75% RH ± 5% RH | 6 months | |
| Refrigerated Case | Long term | 5°C ± 3°C | 12 months |
| Accelerated | 25°C ± 2°C/60% RH ± 5% RH | 6 months | |
| Freezer Case | |||
| Long term | – 20°C ± 5°C | 12 months | |
| Storage below -20°C | API that are studied for storing temperature below -20°C must be handled and evaluated individually, taking into consideration specific circumstances and requirements [62]. | ||
All the above condition for the storage with the stability form can be performed according to the general case, refrigerator and freezer case so on. In order to evaluate the stability of these vesicles, an assessment is conducted on the diameters, zeta potential, and leakiness of the loaded medicines in niosomes over a specific duration of time. Niosomes are more stable than liposomes and have the potential for therapeutic applications.
- In-Vitro Drug diffusion of Niosome: In-vitro drug diffusion of niosomes can be measured using a variety of methods, including: Franz diffusion cell, dialysis bag and spectrophotometry. Franz diffusion cells are a commonly used method for measuring the in-vitro drug diffusion of niosomes [34]. The Franz diffusion cell consists of two chambers separated by a semipermeable membrane. The niosome formulation is placed in the donor chamber and the receptor chamber is packed with a buffer solution. The drug diffusion is measured by sampling the receptor solution at systemic intervals of time and analysing it for the drug concentration.
Franz diffusion cells are a good choice for measuring the in-vitro drug diffusion of niosomes because they are relatively easy to use and provide reproducible results. Additionally, Franz diffusion cells [37] can be used to measure the drug diffusion of niosomes across a variety of semipermeable membranes, which can be useful for predicting the in-vivo drug absorption of niosomes. The semipermeable membrane is located in the middle of donor and receptor chambers within the Franz diffusion cell.
To measure the in-vitro drug diffusion of niosomes using a Franz diffusion cell, the following steps are typically followed:
- The niosome formulation is located in the donor chamber.
- The receptor chamber is packed with a buffer solution.
- The Franz diffusion cell is assembled and placed in an incubator at a constant temperature.
- At regular intervals, samples are taken from the receptor cell and analyzed for the drug concentration.
- The drug diffusion is calculated from the alteration in the drug amount variation in the receptor solution over time [37, 57, 60].
The various evaluation and characterization parameters of the several analgesic niosome previously prepared shown in Table. 10 as below following:
Table. 10: Various characterization and evaluations of analgesic previous prepared with lots of methods
| Drug | Vesicular Size diameter | % Drug Release (hr.) | % Entrapment Efficiency (%EE) | References |
| Aceclofenac | 4.22±0.47µm | 87.21% in 72 h | 96.07%±0.35 | [30] |
| Aceclofenac proniosomes | 136 µm to 236 µm | 45% for 24 h | 97.60±1.85 | [31] |
| Aspirin | — | 45.5% drug release at 3rd hour. | 80.8% | [36] |
| Aspirin | 144.8 ± 12.90 nm | 96.99 ± 1.57% for 24 h | 49 ± 0.15% | [58] |
| Diclofenac sodium | 436±28 nm | 80 % permeation rate | 75±6 % | [57] |
| Diclofenac diethyl ammonium | 224.45 ± 11.95 nm | 32 ± 2 ◦c for 6 h | 93% | [56] |
| Etodolac | 2 𝜇m to 4 𝜇m | 94.91 % in 24 h | 96.72% | [55] |
| Flurbiprofen | 1.35 ± 0.23 𝜇m | – | 88 ± 2.50% | [64] |
| Ketoprofen | 5.24 𝜇m | 64.69 in 12 h | 82.68% | [43] |
| Ketorolac | 4.4±0.21 𝜇m | 203.1±1.6 𝜇g/cm2 h-1 | 99.2±5.2 % | [44] |
| Lornoxicam | 295 nm to 1298 nm | 25.5±4.32% for 24 h | More than 66% | [60] |
| Meloxicam | 70 ± 5.5 nm | – | 84.35 ± 6.8 % | [59] |
| Meloxicam | 107.2±0.6 nm | – | 98% | [26] |
| Nimesulide | 13.4821 𝜇m | 48.1% in 8 hr | 55.89% | [37] |
| Naproxen | 393.9 nm | 88.9 ± 0.71% in 12 h | 95.86%, | [34] |
| Paracetamol | 242.3 ± 18.5 nm | 80% in 8 h | 27.74 ± 4.20% | [65] |
| Piroxicam | 4.81 ± 1.1 𝜇m | 49.38 ± 1.4 𝜇g cm-2 h-1 | 91.7 ± 6.2 % | [66] |
| Tenoxicam | – | 0.61 mg/h | 92% | [45] |
- Separation of Un-entrapped Drug: There are a number of methods that can be used to separate un-entrapped drug from niosomes, including dialysis, gel filtration chromatography, and centrifugation.
- Dialysis: Process of separating molecules depending on their size. Niosomes are typically larger than un-entrapped drug molecules, so dialysis can be used to separate them. To do this, the niosome dispersion is placed in a dialysis bag and the dialysis bag is immersed in a buffer solution. The un-entrapped drug will diffuse through the pores of the dialysis bag into the buffer solution, while the niosomes will remain in the dialysis bag. The un-entrapped drug can then be collected from the buffer solution.
- Gel filtration chromatography (GFC): GFC separates the molecules depending on their sizes. To separate un-entrapped drug from niosomes using gel filtration chromatography, the niosome dispersion is passed through a column filled with a porous material. The niosomes will elute from the column first, followed by the un-entrapped drug. The un-entrapped drug can then be collected from the eluate.
- Centrifugation: Centrifugation is a process of separating particles based on their density. Niosomes are typically denser than un-entrapped drug molecules, so centrifugation can be used to separate them. To do this, the niosome dispersion is centrifuged at a high speed. The niosomes will pellet at the bottom of the centrifuge tube, while the un-entrapped drug will remain in the supernatant. The un-entrapped drug can then be collected from the supernatant [65-67].
- THE APPLICATIONS OF NIOSOME FOR AN ANALGESICS
Analgesics play a vital role in niosomal formulations, as they can be used to relieve pain and inflammation at the targeted site of action. Niosomes are non-ionic surfactant vesicles that are similar to liposomes in structure and function, but offer several advantages, such as greater stability, higher drug loading capacity, and enhanced targeting ability. Analgesic drug niosomes are non-ionic surfactant vesicles that are loaded with analgesic drugs [68]. They are a promising drug delivery system for the treatment of pain and inflammation. Analgesic drug niosomes can be administered orally, ocular, dermal, periodontal or parenterally route. When administered orally, niosomes are absorbed in the intestine and enter the bloodstream. When administered rectally, niosomes are absorbed through the rectal mucosa and enter the bloodstream. When administered topically, niosomes are absorbed through the skin as they have smaller size that makes the permeation easier that leads to enhanced permeation and retention (EPR). When administered parentally, niosomes directly enters into the bloodstream and focus only on the targeted site of the drug [68, 69].
Niosome broadly used in the treatment in pain relieve of the injury mainly focuses on COX (Cyclooxygenase) inhibitors. Cyclooxygenase is involved in various chemical production within the body, one of them is prostaglandins. Prostaglandins is an active lipid based compound (eicosanoids) that mostly concern with the uterine contraction, pain, injury or post injury inflammatory within the body. Flurbiprofen (FBP) is a non-steroidal anti-inflammatory (NSAIDs) that inhibits cyclooxygenase that has currently gained a lot of attention in ocular based drug delivery system [64]. Diclofenac is an anti- inflammatory, antipyretic and analgesic drug. It blocks prostaglandin synthesis and also blocks COX-2 selectively. It is employed to treat bursitis, rheumatoid arthritis, osteoarthritis and ankylosing spondylitis. The drug has a short half-life for 2 hr. and its 70-80% of dose is excreted via renal transport that makes the dosage frequency of Diclofenac more than once a day [57]. Tenoxicam is also a NSAID that is mostly used to the treat rheumatologic based disease and distinguished by its enhanced efficacy and reduced side effects as contrast to supplementary NSAIDs [45]. Niosome formulation improve the permeability of the formulation and prolong as well as sustain the drug release with prolonging the retention time in at specific target area at therapeutic level.
The niosome formulation decrease the frequency of administration and increase the patient compliance. They majorly decrease the side effects for the particular drug interactions.
The main purpose of niosome formulation before the conventional dosage forms is poor bioavailability, Ist pass metabolism and rapid elimination. By using the niosome formulation these drawbacks are reduced and enhances the bioavailability of the drug, providing the localized drug delivery to ensure a targeted action of the drug [66-68]. Formulations has been made for analgesic drugs as Niosomal entrapped drug delivery system. Aceclofenac via ether injection technique with compositions of cholestrol, Span 20, Span 60, Diethyl ether, methanol, PBS of pH 7.4 [30]. The present study investigated the efficacy of formulations of Aspirin via the ether injection method, utilizing a composition consisting of Span 60, cholesterol, diethyl ether, ethanol, and buffer [36]. Additionally, the study evaluated the effectiveness of Diclofenac Sodium as a transdermal niosomal drug delivery system, utilizing the hydration of lipidic film method and a composition of Tween 60, Span 60, and Pluronic F127 [57]. Furthermore, the study examined the potential of Flurbiprofen as an ocular gel, utilizing the TFH method and a composition of Span 60, Carbopol, Carrageenan, and cholesterol [64]. The various niosomal formulations for analgesics with various excipients involving in it as below Table. 11 following discussion.
Table. 11: The list of niosomal formulations of analgesics drugs with including their components and methods involved
| Drug medicament | Methods of formulation | Components | Dosage form | References |
| Aceclofenac | EIM | Cholesterol, Span 20, Span 60, methanol, diethyl ether and PBS and pH 7.4 | Niosome | [30] |
| Aceclofenac proniosomes | Coacervation phase separation method | Cholesterol, Span 20, Span 40, Span 60 | Gel | [31] |
| Aspirin | EIM | Span 60, cholesterol, diethyl ether, ethanol, buffer. | Niosomes | [36] |
| Aspirin | Solvent- based co-precipitation method with a phase inversion technique | Tween 20, PEG 6000, Ethanol, water | Biomimetic niosomal nanoparticles (BNNs) | [58] |
| Diclofenac Sodium | TFH Method | Span 60, Tween 60 and Pluronic F127 | Transdermal niosomes | [57] |
| Diclofenac diethyl ammonium | THF Method | Span 20, 80, Tween 20, 80, Cholesterol, Diethyl ether, chloroform and methanol. | Niosomal formulation | [56] |
| Etodolac | THF Method | Span 60 | Topical Niosomal Gel | [55] |
| Flurbiprofen | THF Method | Span 60 | Ocular gel | [64] |
| Ketoprofen | Modified literature method | Span 80, soya lecithin, Oleic acid, Cholestrol | Periodontal Proniosomes | [43] |
| Ketorolac | Modified literature method | Span 60, Tween 20, soya lecithin, Cholestrol | Transdermal Gel based Proniosomes | [44] |
| Lornoxicam | Thin film hydration technique | Span 40, Dicetyl Phosphate, Stearylamine (SA) | Transdermal Gel based Delivery | [60] |
| Meloxicam | Cold method | Span 60, Poloxamer, Chitosan, Carbopol | Transdermal Gel based Delivery | [59] |
| Meloxicam | TFH Method | Span 60, Carboxy methylcellulose sodium | Transdermal Gel based Delivery | [26] |
| Nimesulide | TFH Method and EIM | Span 20, 40, 60, cholesterol, chloroform, methanol, PBS pH-7.4 | Niosomal formulation | [37] |
| Naproxen | EIM | Span 60, Span 80, Tween 60, Tween 80 | Niosomes | [34] |
| Naproxen | Modified EIM | Tween 80, Brachystegia eurycoma gum, HPMC | Topical Gel based Delivery | [35] |
| Paracetamol (PCM) Antidote | TFH | Span 60, Cholesterol, PEG. | PEGylated nano-niosomes | [65] |
| Piroxicam | Slightly modified method | Span 20, 60, 80, Tween 20, Tween 60, Tween 80 | Transdermal Delivery | [66] |
| Tenoxicam | Modified literature method | Span 20, Span 60, Span 80, Tween 20, Tween 60, Tween 80 | Transdermal proniosomes | [45] |
These are the various drug which prepared via niosomal formulation. Niosomal formulation has potentially benefits to various pharmacological agents for their action against several diseases. Some of their therapeutic benefits are as follows: Gene delivery, Antineoplastic treatment, Delivery of peptide drugs, Drug targeting, studying immune response, Carriers for haemoglobin, Transdermal drug delivery systems, Cosmetics preparations, Anti-Inflammatory (NSAIDs), Anti-Fungal Agents [68-69].
Niosomes can deliver analgesics to the target site of action more effectively than traditional oral or injectable formulations. This is because niosomes can evade the body’s natural defence mechanisms and fuse with cell membranes, releasing the analgesic directly into the cells.
- CONCLUSION
Niosomes are a relatively new drug delivery method comprised of two layers of non-ionic surfactants. Different medications can be put in niosomes by varying the experiment settings and the ratio of surfactant and cholesterol utilised. Furthermore, because niosomes are amphipathic, hydrophobic and hydrophilic medicines may be put into them. Niosomes also improve medication stability, decrease drug toxicity, and delay drug release. Nanoparticles have the potential to serve as a viable substitute for liposomes, thus garnering significant interest within the realm of pharmaceutics. Advanced niosomal formulations, designed for the purpose of theranostics, have the potential to provide valuable insights for diagnostics, treatment, and monitoring of patients’ response to treatment. It is anticipated that the ongoing extensive research dedicated to pioneering niosome formulations will pave the way for the creation of novel pharmaceutical products, thereby enhancing the implementation of the “personalized medicine” approach in the near future. The main purpose of this review to indicate the niosomal preparation in analgesics with their all descriptive data in previous literature.
ACKNOWLEDGEMENT
We would like to thanks all faculty members of Moscow Institute of Physics and Technology, Dolgoprudny, Moscow region, Russia for their collaboration in completion of work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
REFERENCES
- Moammeri A., Masoumeh Mirzaei Chegeni, Hamidreza Sahrayi, et al. “Current advances in niosomes applications for drug delivery and cancer treatment,” Materials Today Bio. pp. 100837-100837, 2023. DOI: https://doi.org/10.1016/j.mtbio.2023.100837
- Yasamineh, S., Yasamineh, P., Kalajahi, H.G., Gholizadeh, O., Yekanipour, Z., Afkhami, H., Eslami, M., Kheirkhah, A.H., Taghizadeh, M., Yazdani, Y. and Dadashpour, M., “A state-of-the-art review on the recent advances of niosomes as a targeted drug delivery system,” International journal of pharmaceutics, vol. 624, pp.121878. 2022, DOI: https://doi.org/10.1016/j.ijpharm.2022.121878
- Masjedi, M. and Montahaei, T., “An illustrated review on nonionic surfactant vesicles (niosomes) as an approach in modern drug delivery: Fabrication, characterization, pharmaceutical, and cosmetic applications,” Journal of Drug Delivery Science and Technology, vol. 61, pp.102234. 2021, DOI: https://doi.org/10.1016/j.jddst.2020.102234
- Bhardwaj, P., Tripathi, P., Gupta, R. and Pandey, S., “Niosomes: A review on niosomal research in the last decade,” Journal of Drug Delivery Science and Technology, vol. 56, pp.101581, 2020, DOI: https://doi.org/10.1016/j.jddst.2020.101581
- Chen, S., Hanning, S., Falconer, J., Locke, M. and Wen, J., “Recent advances in non-ionic surfactant vesicles (niosomes): Fabrication, characterization, pharmaceutical and cosmetic applications,” European journal of pharmaceutics and biopharmaceutics, vol. 144, pp.18-39, 2019, DOI: https://doi.org/10.1016/j.ejpb.2019.08.015
- Khatoon, M., Shah, K.U., Din, F.U., Shah, S.U., Rehman, A.U., Dilawar, N. and Khan, A.N., “Proniosomes derived niosomes: recent advancements in drug delivery and targeting,” Drug delivery, vol. 24, no. 2, pp. 56-69, 2017, DOI: https://doi.org/10.1080/10717544.2017.1384520
- Durak S, Esmaeili Rad M, Alp Yetisgin A, Eda Sutova H, Kutlu O, Cetinel S, Zarrabi A. Niosomal drug delivery systems for ocular disease—Recent advances and future prospects. Nanomaterials. 2020 Jun 18;10(6):1191.
- Sahoo, S.K., Jain, T.K., Reddy, M.K. and Labhasetwar, V., “Nano-sized carriers for drug delivery,” NanoBioTechnology: BioInspired Devices and Materials of the Future, pp.329-348, 2008, DOI: https://doi.org/10.1007/978-1-59745-218-2_13
- Barenholz, Y.C., “Doxil®—The first FDA-approved nano-drug: Lessons learned,” Journal of controlled release, vol.160, no. 2, pp.117-134, 2012, DOI: https://doi.org/10.1016/j.jconrel.2012.03.020
- Abdelkader, H., Alani, A.W. and Alany, R.G., “Recent advances in non-ionic surfactant vesicles (niosomes): self-assembly, fabrication, characterization, drug delivery applications and limitations,” Drug delivery, vol. 21, no. 2, pp. 87-100, 2014, DOI: https://doi.org/10.3109/10717544.2013.838077
- G, D.B., P, V.L. Recent advances of non-ionic surfactant-based nano-vesicles (niosomes and proniosomes): a brief review of these in enhancing transdermal delivery of drug. Futur J Pharm Sci 6, 100 2020, DOI: https://doi.org/10.1186/s43094-020-00117-y
- Singh, P., Ansari, H. and Dabre, S., “Niosomes-a novel tool for anti-ageing cosmeceuticals,” Journal of pharmaceutical research, vol. 6, no. 10, pp.6691-6703, 2016.
- Das, B., Kumar, B., Begum, W., Bhattarai, A., Mondal, M.H. and Saha, B., “Comprehensive review on applications of surfactants in vaccine formulation, therapeutic and cosmetic pharmacy and prevention of pulmonary failure due to COVID-19,” Chemistry Africa, vol. 5, no. 3, pp.459-480, 2022. DOI: https://doi.org/10.1007/s42250-022-00345-0
- Ritwiset, A., Krongsuk, S. and Johns, J.R., “Molecular structure and dynamical properties of niosome bilayers with and without cholesterol incorporation: A molecular dynamics simulation study,” Applied Surface Science, vol. 380, pp.23-31, 2016, DOI: https://doi.org/10.1016/j.apsusc.2016.02.092
- Puras, G., Mashal, M., Zárate, J., Agirre, M., Ojeda, E., Grijalvo, S., Eritja, R., Diaz-Tahoces, A., Navarrete, G.M., Avilés-Trigueros, M. and Fernández, E., “A novel cationic niosome formulation for gene delivery to the retina,” Journal of Controlled Release, vol. 174, pp. 27-36, 2014, DOI: https://doi.org/10.1016/j.jconrel.2013.11.004
- Primavera, R., Palumbo, P., Celia, C., Cinque, B., Carata, E., Carafa, M., Paolino, D., Cifone, M.G. and Di Marzio, L., “An insight of in vitro transport of PEGylated non-ionic surfactant vesicles (NSVs) across the intestinal polarized enterocyte monolayers,” European Journal of Pharmaceutics and Biopharmaceutics, vol. 127, pp. 432-442, 2018, DOI: https://doi.org/10.1016/j.ejpb.2018.03.013
- Barani, M., Mirzaei, M., Torkzadeh-Mahani, M. and Nematollahi, M.H., “Lawsone-loaded Niosome and its antitumor activity in MCF-7 breast Cancer cell line: a Nano-herbal treatment for Cancer,” DARU Journal of Pharmaceutical Sciences, vol. 26, pp.11-17, 2018, DOI: https://doi.org/10.1007/s40199-018-0207-3
- Berlepsch, H.V., Thota, B.N.S., Wyszogrodzka, M., De Carlo, S., Haag, R. and Böttcher, C., “Controlled self-assembly of stomatosomes by use of single-component fluorinated dendritic amphiphiles,” Soft Matter, vol. 14, no. 25, pp. 5256-5269, 2018, DOI: https://doi.org/10.1039/C8SM00243F
- Tavano, L., Muzzalupo, R., Mauro, L., Pellegrino, M., Andò, S. and Picci, N., “Transferrin-conjugated pluronic niosomes as a new drug delivery system for anticancer therapy,” Langmuir, vol. 29, no. 41, pp.12638-12646, 2013, DOI: https://doi.org/10.1021/la4021383
- Mujeeb, S.A. and Sailaja, A.K., “Formulation of ibuprofen loaded niosomal gel by different techniques for treating rheumatoid arthritis,” Journal of Bionanoscience, vol. 11, no. 3, pp.169-176, 2017, DOI: https://doi.org/10.1166/jbns.2017.1435
- Yasam, V.R., Jakki, S.L., Natarajan, J. and Kuppusamy, G., “A review on novel vesicular drug delivery: proniosomes,” Drug delivery, vol. 21, no. 4, pp.243-249, 2014, DOI: https://doi.org/10.3109/10717544.2013.841783
- Sayyad, N., Maji, R., Omolo, C.A., Ibrahim, U.H., Pathan, T.K., Devnarain, N., Karpoormath, R., Dhawan, S., Obakachi, V.A., Merugu, S.R. and Kayamba, F., “Development of niosomes for encapsulating captopril-quercetin prodrug to combat hypertension,” International Journal of Pharmaceutics, vol. 609, pp.121191, 2021, DOI: https://doi.org/10.1016/j.ijpharm.2021.121191
- Sun, Z., “Optimization of clobetasol propionate loaded niosomal gel for the treatment of psoriasis: Ex vivo and efficacy study,” Journal of Dispersion Science and Technology, vol. 44, no. 14, pp.2599-2609, 2023. DOI: https://doi.org/10.1080/01932691.2022.2110111
- Jacob, S., Nair, A.B. and Al-Dhubiab, B.E., “Preparation and evaluation of niosome gel containing acyclovir for enhanced dermal deposition,” Journal of liposome research, vol. 27, no. 4, pp.283-292, 2017. DOI: https://doi.org/10.1080/08982104.2016.1224897
- SURİYAPRAKASH, T.N.K., PARTHİBAN, S., PRABU, S.L. and SUMATHİ, A., “Formulation and evaluation of paclitaxel niosome for its improved anti-cancer activity,” ACTA Pharmaceutica Sciencia, vol. 53, no. 3, 2011.
- El-Badry, M., Fetih, G., Fathalla, D. and Shakeel, F., “Transdermal delivery of meloxicam using niosomal hydrogels: in vitro and pharmacodynamic evaluation,” Pharmaceutical development and technology, vol. 20, no. 7, pp.820-826, 2015. DOI: https://doi.org/10.3109/10837450.2014.926919
- Abou-Taleb, H.A., Khallaf, R.A. and Abdel-Aleem, J.A., “Intranasal niosomes of nefopam with improved bioavailability: preparation, optimization, and in-vivo evaluation,” Drug design, development and therapy, vol. 12, no. 2018, pp.3501-3516, 2018. DOI: https://doi.org/10.2147/DDDT.S177746
- El-Menshawe, S.F. and Hussein, A.K., “Formulation and evaluation of meloxicam niosomes as vesicular carriers for enhanced skin delivery,” Pharmaceutical Development and Technology, vol. 18, no. 4, pp. 779-786, 2013. DOI: https://doi.org/10.3109/10837450.2011.598166
- Tavano, L., Picci, N., Ioele, G. and Muzzalupo, R.J.J.D., “Tetracycline-niosomes versus tetracycline hydrochlo-ride-niosomes: how to modulate encapsulation and percutaneous permeation properties,” J Drug, vol. 1, no. 2, pp. 1-6, 2017. DOI: https://doi.org/10.24218/jod.2017.6
- Srinivas, S., Kumar, Y.A., Hemanth, A. and Anitha, M., “Preparation and evaluation of niosomes containing aceclofenac,” Dig J Nanomater Bios, vol. 5, no. 1, pp. 249-254, 2010.
- Radha, G.V., Sastri, K.T., Prathyusha, P., Bhanu, P. and Rajkumar, J., “Formulation and evaluation of aceclofenac proniosome loaded orabase for management of dental pain,” Int J Appl Pharm, vol. 10, no. 7, pp. 204-10, 2018. DOI: : http://dx.doi.org/10.22159/ijap.2018v10i6.29143
- Kumar, A. and Dua, J.S., “Formulation and evaluation of itraconazole niosomal gel,” Asian Journal of Pharmaceutical Research and Development, 6(5), pp.76-80, 2018. DOI: https://doi.org/10.22270/ajprd.v6i5.425
- Umbarkar, M., Thakare, S., Surushe, T., Giri, A. and Chopade, V., “Formulation and evaluation of liposome by thin film hydration method,” Journal of drug delivery and therapeutics, vol. 11, no. 1, pp. 72-76, 2021. DOI: https://doi.org/10.22270/jddt.v11i1.4677
- Sailaja, A.K. and Shreya, M., “Preparation and characterization of naproxen loaded niosomes by ether injection method,” Nano Biomedicine and Engineering, vol. 10, no. 2, pp. 174-180, 2018. DOI: https://doi.org/10.5101/nbe.v10i2.p174-180
- Okafo, S.E., Ordu, J.I., Ofagbor, G. and Agbamu, E., “Evaluation of physicochemical, in vivo analgesic and antiinflammatory activities of Brachystegia eurycoma gum-based naproxen loaded niosomal gels,” German Journal of Pharmaceuticals and Biomaterials, vol. 2, no. 1, pp.26-37, 2023. DOI: https://doi.org/10.5530/gjpb.2023.1.3
- Krishna Sailaja A and Ruth John C. “Aspirin Loaded Niosomes A Novel Drug Delivery System by Ether Injection Method,” Nanomed Nanotechnol, vol. 8, no. 3, pp. 000237, 2023. DOI: https://doi.org/10.23880/nnoa-16000237
- Singh, C.H., Jain, C. and Kumar, B.N., “Formulation, characterization, stability and invitro evaluation of nimesulide niosomes,” Pharmacophore, vol. 3, no. 3, pp.168-85, 2011. https://pharmacophorejournal.com/E7JaDiu
- Negi, P., Aggarwal, M., Sharma, G., Rathore, C., Sharma, G., Singh, B. and Katare, O.P., “Niosome-based hydrogel of resveratrol for topical applications: An effective therapy for pain related disorder (s),” Biomedicine & Pharmacotherapy, vol. 88, pp. 480-487, 2017. DOI: https://doi.org/10.1016/j.biopha.2017.01.083
- Sankhyan, A. and Pawar, P., “Recent Trends in Niosome as Vesicular DrugDelivery System,” Journal of Applied Pharmaceutical Science, vol. 2, no. 6, pp.20-32, 2012. DOI: http://dx.doi.org/10.7324/JAPS.2012.2625
- Jothy, M.A. and Shanmuganathan, S., “An overview on niosome as carrier in dermal drug delivery” Journal of pharmaceutical sciences and research, vol. 7, no. 11, pp. 923, 2015.
- Izhar, M.P., Hafeez, A., Kushwaha, P. and Simrah, “Drug Delivery Through Niosomes: A Comprehensive Review with Therapeutic Applications” Journal of Cluster Science, pp.1-17, 2023. DOI: https://doi.org/10.1007/s10876-023-02423-w
- Chen, S., Hanning, S., Falconer, J., Locke, M. and Wen, J., “Recent advances in non-ionic surfactant vesicles (niosomes): Fabrication, characterization, pharmaceutical and cosmetic applications,” European journal of pharmaceutics and biopharmaceutics, vol. 144, pp.18-39, 2019. DOI: https://doi.org/10.1016/j.ejpb.2019.08.015
- Yadav, N.K., Nanda, S., Sharma, G. and Katare, O.P., “Systematically optimized ketoprofenloaded novel proniosomal formulation for periodontitis: in vitro characterization and in vivo pharmacodynamic evaluation,” AAPS PharmSciTech, vol. 18, pp.1863-1880, 2017. DOI: https://doi.org/10.1208/s12249-016-0665-1
- Alsarra, I.A., Bosela, A.A., Ahmed, S.M. and Mahrous, G.M., “Proniosomes as a drug carrier for transdermal delivery of ketorolac,” European journal of pharmaceutics and biopharmaceutics, vol. 59, no. 3, pp.485-490, 2005. DOI: https://doi.org/10.1016/j.ejpb.2004.09.006
- Ammar, H.O., Ghorab, M., El-Nahhas, S.A. and Higazy, I.M., “Proniosomes as a carrier system for transdermal delivery of tenoxicam,” International journal of pharmaceutics, vol. 405, no. 1- 2, pp.142-152, 2011. DOI: https://doi.org/10.1016/j.ijpharm.2010.11.003
- Negi, P., Aggarwal, M., Sharma, G., Rathore, C., Sharma, G., Singh, B. and Katare, O.P., “Niosome-based hydrogel of resveratrol for topical applications: An effective therapy for pain related disorder (s),” Biomedicine & Pharmacotherapy, vol. 88, pp. 480-487, 2017. DOI: https://doi.org/10.1016/j.biopha.2017.01.083
- Ge, X., Wei, M., He, S. and Yuan, W.E., “Advances of non-ionic surfactant vesicles (niosomes) and their application in drug delivery,” Pharmaceutics, vol. 11, no. 2, pp. 55, 2019. DOI: https://doi.org/10.3390/pharmaceutics11020055
- Sankhyan, A. and Pawar, P., “Recent Trends in Niosome as Vesicular DrugDelivery System,” Journal of Applied Pharmaceutical Science, vol. 2, no. 6, pp.20-32, 2012. DOI: http://dx.doi.org/10.7324/JAPS.2012.2625
- Jothy, M.A. and Shanmuganathan, S., “An overview on niosome as carrier in dermal drug delivery” Journal of pharmaceutical sciences and research, vol. 7, no. 11, pp. 923, 2015.
- Izhar, M.P., Hafeez, A., Kushwaha, P. and Simrah, “Drug Delivery Through Niosomes: A Comprehensive Review with Therapeutic Applications” Journal of Cluster Science, pp.1-17, 2023. DOI: https://doi.org/10.1007/s10876-023-02423-w
- Shahiwala, A. and Misra, A., “Studies in topical application of niosomally entrapped nimesulide,” J Pharm Pharm Sci, vol. 5, no. 3, pp. 220-225, 2002.
- Sahin, N.O., “Niosomes as Nanocarrier Systems,” In: Mozafari, M.R. (eds) Nanomaterials and Nanosystems for Biomedical Applications. Springer, Dordrecht, 2007, pp. 67-81. DOI: https://doi.org/10.1007/978-1-4020-6289-6_4
- G, D.B., P, V.L. “Recent advances of non-ionic surfactant-based nano-vesicles (niosomes and proniosomes): a brief review of these in enhancing transdermal delivery of drug,” Futur J Pharm Sci 6, 100 2020, DOI: https://doi.org/10.1186/s43094-020-00117-y
- Guo, M., He, Z., He, X. and Song, X., “Surface modification of liposomes using folic acid,” In Liposomes: Methods and Protocols, New York, NY: Springer US., pp. 191-196, 2023. DOI: https://doi.org/10.1007/978-1-0716-2954-3_16
- Shilakari Asthana, G., Asthana, A., Singh, D. and Sharma, P.K., “Etodolac containing topical niosomal gel: formulation development and evaluation,” Journal of drug delivery, volume 2016, article ID 9324567, pp. 1-8, 2016. DOI: http://dx.doi.org/10.1155/2016/9324567
- Manosroi, A., Jantrawut, P. and Manosroi, J., “Anti-inflammatory activity of gel containing novel elastic niosomes entrapped with diclofenac diethylammonium,” International journal of pharmaceutics, vol. 360, no. 1-2, pp.156-163, 2008. DOI: https://doi.org/10.1016/j.ijpharm.2008.04.033
- Tavano, L., de Cindio, B., Picci, N., Ioele, G. and Muzzalupo, R., “Drug compartmentalization as strategy to improve the physico-chemical properties of diclofenac sodium loaded niosomes for topical applications,” Biomedical microdevices, vol. 16, pp.851-858, 2014. DOI: https://doi.org/10.1007/s10544-014-9889-6
- Rajeev, J., Kamalasanan, K., Sapa, H., Sabitha, M. and Abhi, C., “Controlled release nanomedicine (CRNM) of aspirin using “biomimetic niosomal nanoparticles (BNNs)” for Covid-19 and cardiovascular treatment: DOE based optimization,” OpenNano, vol. 9, pp.100119, 2019. DOI: https://doi.org/10.1016/j.onano.2022.100119
- El-Badry, M., Fetih, G., Fathalla, D. and Shakeel, F., “Transdermal delivery of meloxicam using niosomal hydrogels: in vitro and pharmacodynamic evaluation,” Pharmaceutical development and technology, vol. 20, no. 7, pp.820-826, 2015. DOI: https://doi.org/10.3109/10837450.2014.926919
- . El-Ridy, M.S., Yehia, S.A., Mohsen, A.M., El-Awdan, S.A. and Darwish, A.B., “Formulation of niosomal gel for enhanced transdermal lornoxicam delivery: in-vitro and in-vivo evaluation,” Current Drug Delivery, vol. 15, no. 1, pp.122-133, 2018. DOI: https://doi.org/10.2174/1567201814666170224141548
- Ge, X., Wei, M., He, S. and Yuan, W.E., “Advances of non-ionic surfactant vesicles (niosomes) and their application in drug delivery,” Pharmaceutics, vol. 11, no. 2, pp. 55, 2019. DOI: https://doi.org/10.3390/pharmaceutics11020055
- Moghassemi, S. and Hadjizadeh, A., “Nano-niosomes as nanoscale drug delivery systems: an illustrated review,” Journal of controlled release, vol. 185, pp.22-36, 2014. DOI: https://doi.org/10.1016/j.jconrel.2014.04.015
- Akbarzadeh, I., Sedaghatnia, K., Bourbour, M., Moghaddam, Z., Moghtaderi, M., Samimi-Sohrforozani, E., Quazi, S. and Far, B., “Niosomes: a novel targeted drug delivery system,” 2021.
- El-Sayed, M.M., Hussein, A.K., Sarhan, H.A. and Mansour, H.F., 2017. Flurbiprofen-loaded niosomes-in-gel system improves the ocular bioavailability of flurbiprofen in the aqueous humor. Drug development and industrial pharmacy, vol. 43, no. 6, pp.902-910, 2017. DOI: https://doi.org/10.1080/03639045.2016.1272120
- Firozian, F., Karami, S., Ranjbar, A., Azandaryani, M.T. and Nili-Ahmadabadi, A., “Improvement of therapeutic potential N-acetylcysteine in acetaminophen hepatotoxicity by encapsulation in PEGylated nano-niosomes,” Life Sciences, vol.n 255, pp.117832, 2020. DOI: https://doi.org/10.1016/j.lfs.2020.117832
- Gad, S., Ahmed, A.M., Ghourab, M.M. and Queshawy, M.K., “Design, formulation, and evaluation of Piroxicam niosomal gel,” Int. J. PharmTech. Res, vol. 6, no. 1, pp. 185-195, 2014.
- Muzzalupo, R., Tavano, L., Cassano, R., Trombino, S., Ferrarelli, T. and Picci, N., “A new approach for the evaluation of niosomes as effective transdermal drug delivery systems,” European Journal of Pharmaceutics and Biopharmaceutics, vol. 79, no. 1, pp.28-35, 2011. DOI: https://doi.org/10.1016/j.ejpb.2011.01.020
- Qumbar, M., Imam, S.S., Ali, J., Ahmad, J. and Ali, A., “Formulation and optimization of lacidipine loaded niosomal gel for transdermal delivery: in-vitro characterization and in-vivo activity,” Biomedicine & Pharmacotherapy, vol. 93, pp.255-266, 2017. DOI: https://doi.org/10.1016/j.biopha.2017.06.043
- Obeid, M.A., Khadra, I., Mullen, A.B., Tate, R.J. and Ferro, V.A., “The effects of hydration media on the characteristics of non-ionic surfactant vesicles (NISV) prepared by microfluidics,” International journal of pharmaceutics, vol. 516, no. 1-2, pp.52-60, 2017. DOI: https://doi.org/10.1016/j.ijpharm.2016.11.015
