Introduction
There are a variety of furan derivatives which were developed by linking N-heterocycles with carbohydrazides as a new scaffold of biological agents. The development of new efficient methods to synthesize N-heterocycles with structural diversity is one major interest of synthetic organic chemists. The utilization of 2(3H)-furanones for the construction of a wide variety of nitrogenous heterocyclic ring systems of synthetic and biological importance had been a subject of research concern [1-7]. These diverse biological activities initiated our interest in utilizing the 2(3H)-furanone bearing a furyl moiety as side chain for the synthesis of nitrogen containing heterocycles of anticipated biological activities.
Results and Discussion
Furyl-2(3H)-furanone 3 was previously prepared [8] as mixture of (E) and (Z) stereoisomers via condensation of 3-benzoylpropionic acid 1 with furan-2-carbooxaldehyde 2 in the presence of thionyl chloride/N,N-dimethylformamide mixture as a cyclodehydrating agent [9,10] (Scheme 1).
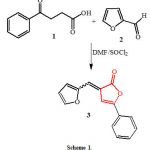 |
Treatment of 2(3H)-furanone 3 with phenylhydrazine and ethylenediamine was found to be dependent on the reaction conditions. Indeed, stirring a solution of 3 in ethanol with phenylhydrazine or ethylenediamine at room temperature afforded the open chain N-phenylhydrazide derivative 4 and amide derivative 6, respectively (Scheme 2). The reaction could be explained via the nucleophilic attack of NH2 on the C=O of furanone nucleus to lead to ring opening giving the acyclic products.
On the other hand, carrying out the reaction at refluxing conditions in the same solvent led to the construction of pyridazinone 5 and pyrrolone 7 derivatives (Scheme 2). The structures of all compounds 4-7 were inferred from their analytical as well as spectral data. The IR spectra of all products were lacked to n C=O of the starting furanone which was at 1766 cm-1. The 1H-NMR spectra exhibited the labile hydrogen atoms and disappeared with D2O (Cf. Experimental). The reaction could be postulated via lactone ring opening by nucleophilic attack of NH2 on the C=O of furanone nucleus followed by ring closure.
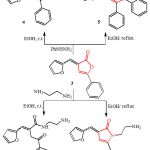 |
Conclusion
In this work, we designed and synthesized some valuable nitrogen containing heterocycles such as pyrrolone and pyridazinone derivatives in addition to acyclic products. The reactions of 2(3H)-furanone with Nitrogen nucleophiles were found to be dependent upon the reaction conditions.
Experimental
Melting points were measured on a Gallenkamp electric melting point apparatus and are uncorrected. The infrared (IR) spectra were recorded using potassium bromide disks on IR Thermo Electron Nicolet 7600 (USA) infrared spectrometer at the Central Laboratory of Faculty of Science, Ain Shams University. The 1H-NMR spectra were run at 400 MHz on a GEMINI 400 BB NMR spectrometer using tetramethyl silane (TMS) as internal standard in deuterated dimethylsulfoxide (DMSO-d6) at the Main Defense Chemical Laboratory, Cairo, Egypt. The reactions were monitored by the thin layer chromatography using Merck Kiesel gel 60 F254 aluminum backed plates.
General procedure for the reaction of furanone 3 with phenylhydrazine or ethylenediamine
To a solution of furanone 3 (2 mmol) in absolute ethanol (20 ml), phenylhydrazine or ethylenediamine (2 mmol) was added with stirring at room temperature. The reaction mixture was stirred for 3 h at room temperature. The precipitated solid was collected by filtration and recrystallized from the suitable solvent to afford the open chain compounds 4 and 6, respectively.
2-(Furan-2-ylmethylene)-4-oxo-N’,4-diphenylbutanehydrazide (4)
White crystals, mp. 220-222oC (Ethanol), yield 68%. Anal. Calcd. for C21H18N2O3 (346.13): C, 72.82; H, 5.24; N, 8.09. Found: C, 72.68; H, 5.10; N, 8.11. IR (n, cm-1): 3331 (NH), 1685,1658 (C=O). 1H-NMR (DMSO-d6): δH (ppm) 10.70 (s, 1H, NHCO, Exchangeable), 7.88-7.27 (m, 13H, Ar-H), 7.15 (s, 1H, CH=), 7.10 (s, 1H, NHPh, Exchangeable), 3.34 (s, 2H, CH2CO).
N-(2-Aminoethyl)-2-(furan-2-ylmethylene)-4-oxo-4-phenylbutanamide (6)
White crystals, mp. 115-117oC (Light Petroleum), yield 74%. Anal. Calcd. for C17H18N2O3 (298.13): C, 68.44; H, 6.08; N, 9.39. Found: C, 68.31; H, 5.94; N, 9.42. IR (n, cm-1): 3359, 3290 (NH, NH2), 1684,1653 (C=O). 1H-NMR (DMSO-d6): δH (ppm) 8.70 (s, 1H, NH, Exchangeable), 7.85-7.21 (m, 8H, Ar-H), 7.12 (s, 1H, CH=), 3.82 (t, 2H, CH2NH, J= 6.5 Hz), 3.37 (s, 2H, CH2CO), 3.45 (t, 2H, CH2NH2, J= 6.3 Hz), 5.21 (br.s, 2H, NH2, Exchangeable).
Formation of the pyridazinone 5 and pyrrolone derivative 7
A mixture of furanone 3 (2 mmol) and phenylhydrazine or ethylenediamine (2 mmol) was heated under reflux in absolute ethanol (20 ml) for 5 h. The precipitated solid after cooling was collected by filtration and recrystallized from the suitable solvent to give pyridazinone 5 and pyrrolone 7, respectively.
4-(Furan-2-ylmethylene)-1,6-diphenyl-1,2-dihydropyridazin-3(4H)-one (5)
White crystals, mp. 254-256oC (Ethanol), yield 61%. Anal. Calcd. for C21H16N2O2 (348.12): C, 76.81; H, 4.91; N, 8.53. Found: C, 76.73; H, 4.79; N, 8.49. IR (n, cm-1): 3350 (NH), 1652 (C=O). 1H-NMR (DMSO-d6): δH (ppm) 10.70 (s, 1H, NH, Exchangeable), 7.89-7.25 (m, 13H, Ar-H), 7.14 (s, 1H, CH=), 6.91 (s, 1H, C-H Pyridazine).
1-(2-Aminoethyl)-3-(furan-2-ylmethylene)-5-phenyl-1H-pyrrol-2(3H)-one (7)
Orange crystals, mp. 240-242oC (Ethanol), Anal. Calcd. for C17H16N2O2 (280.12): C, 72.84; H, 5.75; N, 9.99. Found: C, 72.69; H, 5.68; N, 9.95. IR (n, cm-1): 3326, 3250 (NH2), 1690 (C=O). 1H-NMR (DMSO-d6): δH (ppm) 7.81-7.25 (m, 8H, Ar-H), 6.95 (br.s, 2H, NH2, Exchangeable), 3.53 (t, 2H, CH2N, J= 11.4 Hz), 3.39 (t, 2H, CH2NH2, J= 11.7 Hz).
References
- Ramadan, S. K. and Abou-Elmagd, W. S. I.; Commun. 48(18);2409. 2018.
- Ramadan, S. K.; El-Helw, E. A. E. J. Chem. Res. 42;332.
- Ramadan, S. K.; Hashem, A. I.; Abou-Elmagd, W. S. I.; El-Ziaty, A. K. Heterocycl. Chem, 54;3711. 2017.
- Ramadan, S. K.; El-Ziaty, A. K.; Abou-Elmagd, W. S. I.; Hashem, A. I. Synth. Commun, 47;471. 2017.
- Abou-Elmagd, W. S. I.; El-Ziaty, K.; El-Zahar, M. I.; Ramadan, S. K.; Hashem, A. I. Synth. Commun. 46(14); 1197. 2016.
- Hashem, A. I.; Abou-Elmagd, W. S. I.; El-Ziaty, A. K. and Ramadan, S. K.; Heterocycl. Chem. 54;3711. 2017.
- Ramadan, S. K.; Youssef, A. M.; El-Ziaty, A. K.; Abou-Elmagd, W. S. I. J. Heterocyclic Chem. 52;278. 2015.
- Hashem, A. I. Prakt. Chem. 319; 689. 1977.
- Ramadan, S. K.; El-Ziaty, A. K.; Abou-Elmagd, W. S. I.; Hashem, A. I. J. Chem. 59(4), 637. 2016.
- Khan, R. H.; Rastogi, R. C. Chem. Res. 260. 1991.
