Introduction
Wide applications for Nano Bi2O3 were used as a catalyst, capacitor, additive in paints and gas sensors. Because of the compounds of Bi2O3 has structure face-centered cubic, it has high conductivity compare with all conductors oxides and it is the best solid electrolytes. Four phases of Bi2O3were existed: α, β, γ and e. Many methods were used to prepare it such as sol gel, chemical precipitation and hydrothermal methods. [1-5] In this paper, a new method was studied to prepare the particles called photolysis method. According this method, UV irradiation [6-11] works as a reduction reagent to prepare the nano surface.
Experimental
Characterization
AFM and XRD were used to character the image surface, average and crystal system of the nanoparticles.
Synthesis of Bi2O3 nanoparticles
5 g of bismuth chloride dissolved in 100ml of ethanol and photolysis it using irradiation system as shown in figure 1 for 2hr until brown precipitate appear. After that, burned the precipitate for 1hr at 400C until yellow powder appears.
 |
Figure 1: irradiation system (125W)
|
Result and Discussion
The average of particles appeared in figure 2. The results show prepare particles in nano range.
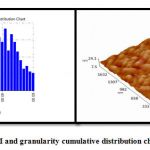 |
Figure 2: 3D image of AFM and granularity cumulative distribution chart for a-Bi2O3
|
The crystal size of nanoparticles was determined by using Debye-Scherrer formula by following equation [12]:
D = k λ / βCosθ
Where λ is the wavelength of the Cu-Kα radiations, k scherrer constant, β is the full width at half maximum and θ is the angle obtained from 2θ values corresponding to maximum intensity peak in XRD pattern. The result from equation show prepare particle in nano size (30nm).
The structure of alpha phase of bismuth oxide nanoparticles was characterized by using XRD technique that prepared by photolysis methods. Fig 3 was shown the pattern of alpha phase and miller index of the powder and it showed that the nanoparticles have monoclinic structure according standard card (JCPDS 00-041-1449).
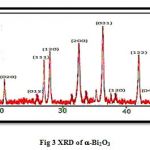 |
Conclusion
By simple photolysis method, bismuth oxide nanoparticles have been successfully prepared. The checking of the AFM and XRD were showing us the composition of a-Bi2O3 with 38nm.
Reference
- Devi, G., S. Manorama., and V. Rao. SnO2:Bi2O3 based CO sensor: laser-Raman, temperature programmed desorption and X-ray photoelectron spectroscopic studies, Sens. Actuators B, 56 (1/2) 98-105 ;1999.
- Geetha, M., K. Suguna., P. Anbarasan., and V. Aroulmoji. International Journal of Advanced Science and Engineering. 1(1), 1-5; 2014.
- Han-pei, Y., F. Yi-ning., L. Ming., X. Bo-lian., and C. Yi. Structure and catalytic properties of Bi−Mo composite oxide catalyst for selective oxidation of propane. Journal of 60(6): 1006−1010; 2002 .
- Laurent, K., G. Wang., T. Nenezs., and Y. LEPRINCE. Structure and conductivity studies of electrodeposited δ-Bi2O3. Journal of Solid State Ionics: Diffusion and Reactions, 178(33): 1735−1739; 2008.
- Lazarevic, Z., B. Stojanovic., and J. Varela. An approach to analyzing synthesis, structure and properties of bismuth titanate ceramics. Journal of Science of Sintering, 37(3): 199−216; 2005.
- Zaid, H.M; Nuha, F.A; Aklas, A.A., Effect of Solvents on the Size of Copper Oxide Particles Fabricated using Photolysis Method Asian J. Chem., 30 (2018), 223-225.
- Ahmed, N; Zaid, H.M., Synthesis of a-Fe2O3 Nano Powders by Novel UV Irradiation Method, DJPS., 14: 56-66; 2018.
- Mohammed, A.F; Zaid, H.M; Marwa, S.F. Syntheses, characterization and studying TiO2/Au nanocomposite via UV-irradiation method and its effective to degradation of methylene blue, Asian J. Chem., 30, 1142-1146; 2018.
- Zaid, H.M. The Magnetic Properties of Alpha Phase for Iron Oxide NPs that Prepared from its Salt by Novel Photolysis Method, J Chem Pharm Res., 9, 29-33;2017 .
- Zaid H.M., Effect of Au doping on the Magnetic Properties of Fe3O4 NPs Prepared via Photolysis and co-Precipitation Methods, DJPS., 14, 136-146; 2018.
- Zaid H.M., Synthesis of Bismuth oxide Nano powders viaelectrolysis method and study the effect of change voltage on the size for it, Australian Journal of Basic and Applied Sciences, 11(7), 97-101; 2017.
- Ahmad, M., A. Rahdar., F. Sadeghfar., S. Bagheri., and M. Hajinezhad. Journal of Nanomed Res, 2016, 1(1), 39-46; 2016.
