Introduction
Blackberry (Rubus fruticosus) is an important economic and medicinal purpose fruit. It is a widespread and well known perennial shrub under Rosaceae family with fruits of dark red to black in color (Livani et al., 2013). Fruit is produced on two year old cane and when mature, the plant can reach up to 10 feet tall. It prefers well-drained, loamy and moist soils. Europe, North America, Asia, South America, Oceania Central America, and Africa are the main regions for blackberry production. Commercial production of blackberry in the world is estimated to be approximately 154,578 tons annually (Kaume et al., 2011).
Blackberry fruits are an excellent source of natural antioxidants, which is one of the major reasons for their increasing popularity in the human diet. It is also a fruit of interest because of its high content of anthocyanins with attractive color. Many research reports suggest that consumption of anthocyanins may decrease the risk of obesity, coronary heart disease, degenerative conditions, and various types of cancer (Barros et al., 2010).
Importance of the anthocyanin for the beneficial health effects associated with their antioxidant, anti-inflammatory, and chemo-preventative properties; the biological activity of blackberry against esophageal, colon, and oral cancers has been demonstrated (Freds et al., 2014). It has long been established that cyanidin-3-glucoside and cyanidin-3-rutinoside are the respective major and minor anthocyanin’s in blackberries (Fan-Chiang and Wrolstad, 2005). Fruit-based dietary supplements also targeted to increase consumers’ anthocyanin intake has risen recently.
Blackberry extracts have also exerted antimutagenic effects in vitro and in vivo by modifying cell signaling pathways and suppressing tumor promotion factors. However, the anti-obesity, antidiabetic, antimicrobial, and anti-inflammatory properties of blackberry phenolic compounds need investigation. Similarly, studies that elucidate the in vivo physiologically effective concentrations of blackberry phenolic compounds are necessary.
Blackberry leaves are used in making blackberry tea and used for treating non-specific acute diarrhea, as well as in inflammation of the mouth and throat. It is also reported to be helpful in reducing blood sugar levels and is a good source of the vitamin C and E. Root of blackberry is also used for different purposes.
Blackberry fruit is populating day by day in the world due to high content of anthocyanin. But its production is not satisfactory in Asia though climate is very favorable for blackberry cultivation in the some regions of Asia. Blackberry, being easy to handle and having low production costs even concerning chemicals, is an alternative crop for agriculture (Hassimoto et al., 2008). There is no commercial blackberry production in Japan. For the high fruit values, a few Japanese people are cultivating it in a small scale at their house area. Fruit size-shape, color, and taste are also good. Commercial blackberry production in Japan may play important role for its economy. A very few researches have been done on blackberry in Japan.
Therefore, due to high fruit and economic values of blackberries, the present research work has been taken for the identification of anthocyanin from the blackberry fruit in Japan for commercial application.
Materials and Methods
Standard preparation
The standard solution was prepared by accurately weighing 5 mg of blackberry, transferring it to a 5 mL volumetric flask, and making up the volume using methanol in order to make 1 mg/mL solution.
Extraction of sample
Fresh and mature blackberry fruits were collected from near Okayama University. The sample was prepared by accurately weighing 10 g of fruits and extracting it with methanol by sonication. The extracts were filtered through filter paper and evaporated. Finally, the extracted fruit solution was used for HPLC analysis and NMR.
HPLC analysis
HPLC was performed using Jasco Co. Ltd. Solvent ratio was multilinear gradient produced with A (acetonitrile) and B (0.5% TFA). Gradient elution was as follows: from 0 to 20% A in 25 min, from 20 to 30% A in 60 min and column: CrestPak C18S (150 × 4.6 mm). The flow rate was 1 mL/minute, and the detector was set at 510 nm. The injection volumes were 5 μL throughout the experiment. Anthocyanin in the samples was identified using HPLC by measuring the retention time.
NMR analysis
1H NMR was analyzed 400 MHz from JEOL JNM-ESC400. Solvent was methanol-d4 .
Results and Discussion
Anthocyanins have recently received increasing attention as natural colorants in food systems, as against synthetic ones. Scientist are working with this valuable pigment throughout the world to pay attention its high food values. In the present experiment, we tried to detect anthocyanin from fresh blackberry fruits.
Various analytical methods have been reported for profiling anthocyanin content of fruits. The common approach includes a combination of liquid chromatography-based separation with light absorbance-based quantification (Aaby, Wrolstad, Ekeberg, & Skrede, 2007; Kahle et al., 2006), usually at 520 nm wavelength, and nuclear magnetic resonance (NMR) spectroscopy for structural identification. The most abundant anthocyanins in the edible parts of plants are cyanidin, followed bypelargonidin, peonidin,delphinidin, petunidin, and malvidin (Hager, Howard, Prior, & Brownmiller, 2008).
 |
Figure 1: HPLC profile of the EtOAc extract fraction (A) and n-BuOH extract fraction (B) |
From our present experiment, we detected three peaks from sample using 520 nm wavelength. Cyanidin-3-glucoside standard detected Rt=30 min by HPLC analysis. After comparison with standard, we confirmed peak 1 is cyanidin-3-glucoside (Figure 1). Cyanidin-3-glucoside from blackberry fruits was detected by same retention time (Bowen-Forbesa 2010, Zhang et al. 2012 and Jin et al. 2016).
In case of NMR data, peak 1 isolated from sample of fraction B was analyzed. According to NMR data, we concluded that peak 1 is cyanidin-3-glucoside. NMR data clearly showed the presence of only one cyanidin 3-glucoside. Two papers (Mcghie et al., 2006 and Zarina et al., 2012) reported the identification of cyaniding-3-glucoside by NMR. Linkage showed that all the sugar units are linked to each other. It suggests that the chemical structure of anthocyanin-conjugated sugar may affect its thermal stability and thus may affect their antioxidant activity.
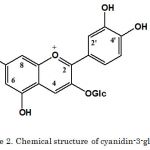 |
Figure 2: Chemical structure of cyanidin-3-glucoside
|
Conclusion
The major objective of the present study was to investigate the presence of anthocyanin pigments in blackberry, elucidating the chemical structures by NMR spectroscopy and HPLC. We successfully detected cyanidin-3-glucoside pigments from blackberry fruits. This result can play important role for commercial blackberry production in Japan which contain high economic value. Further experiment will be taken for more understanding on anthocyanin.
References
- Aaby, Wrolstad, Ekeberg, & Skrede, Polyphenol composition and antioxidant activity in strawberry pures ; impact of achene level and storage. J of Agri and Food Chem 2007 55.5156-5166
- Barros L, Oliveria S, Carvalho AM and Ferreira ICFR In vitro antioxidant properties and characterization in nutrients and phytochemicals of six medicinal plants from the Portuguese folk medicine. Ind Cross Prod 2010 32 572-579.
- Bowen-Forbesa C S, Zhang Y, Nair M G Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J of food Com and Analysis 2010 23 6 554-560
- Fan-Chiang, H.J Wrolstad, R.E Food Sci.2005, 70,198.
- Hassimoto N M A, Mota R V, Cordenunsi B R and Lajolo F M Physico-chemical characterization and bioactive compounds of blackberry fruits Rubus sp grown in Brazil Cienc, Technol. Aliment Campinas 2008 28 3 702-708
- Hager A,Howard LR, Prior RL and Brownmiller CR Processing and storage effects on monomeric anthocyanins, percent polymeric colour and antioxidant capacity of processed black rasbebby products. J of Food Sci 200873 H134-140
- Jin YH, Shin HJ, Ahn HJ, Jang BK, Park NH, and Hong JIdentification of Anthocyanin’s in Berry Species Using LC-ESI-MS Combined with High-Speed Counter-Current Chromatography 2016 Bull. Korean Chem. Soc. 2016, Vol. 37, 1618–1624
- Kaume L, Luke R H and Devareddy L The Blackberry Fruit: A Review on Its Composition and Chemistry, Metabolism and Bioavailability, and Health Benefits J Agric Food Chem 2012 60 5716-5727
- Kahle et al., Studies on apple and blueberry fruit constituents: Do the polyphenols reach the colon after ingestion? Molecular Nutrient and Food Research 2006 50:418-423.
- Livani F, Ghorbanli M and Sateeyi A Changes in antioxidant activity and content of phenolic compounds during the riening process of elm-leaved blackberry fruit I.J of Agro and P pro 2013 vol 4188-93.
- Mazza, G and Minitiati Introduction. In; Anthocyanin in fruits, Vegetables and Grains. Boca rattan FL CRC Press Chapter1’ 1-28.
- Moyer RA, Hummer KE, Finn CE, Frei B, Wrolstad RE Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, rubus, and ribes. J Agric Food Chem 2001 50(3):519–525.
- Mcghie T K, Rowan D R and Edwards P J Structural Identification of Two Major Anthocyanin Components of Boysenberry by NMR Spectroscopy J Agric Food Chem 2006 54;8756-8761.
- Zhang L,Zhou J, Liu H, Khan M A,Huang K and Gu Z Compositions of anthocyanins in blackberry juice and their thermal degradation in relation to antioxidant activity Eur Food Res Techno (2012) 235:637–645.
- Zariana A S and Sankar K U Isolation and identification of pelargonidin 3-glucoside in Mangosteen pericarp. Food Chem 2012 130A 665-670.
